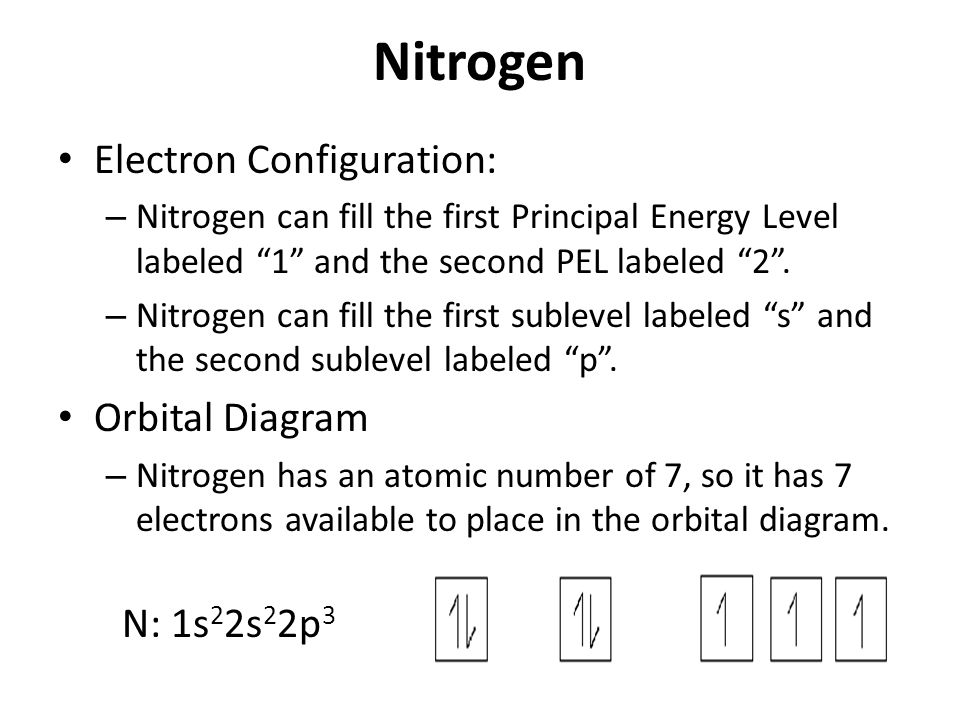
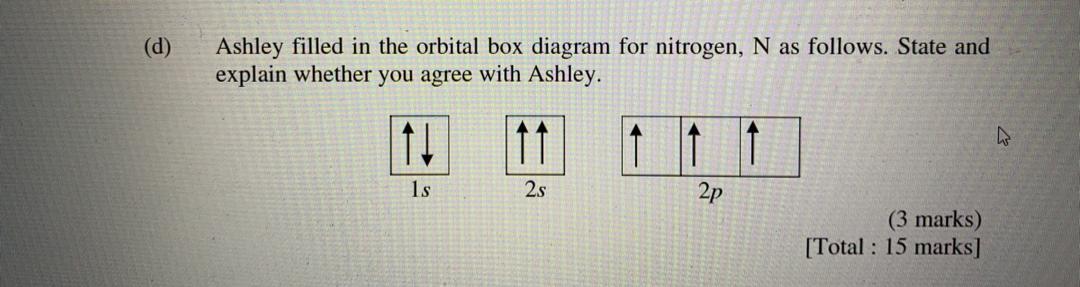
41 orbital diagram of nitrogen
NO2 Lewis Structure, Molecular Geometry, Hybridization ... Lewis Structure of NO2. A molecule of nitrogen dioxide consists of one nitrogen atom and two atoms of oxygen. Let us look at the periodic table. Nitrogen belongs to group 15 ( or group 5) and has an atomic number of 7, therefore has a valency of 5. Oxygen belongs to group 16 ( or group 6) and has an atomic number of 8, therefore a valency of 6. N2+ Mo Diagram - schematron.org The correlation diagrams for nitrogen and carbon monoxide and the first are nearly parallel to the corresponding orbital energy curves. Bond order for N2 is 3; bond order for N2- is and bond order for N2+ is I have not included pictures of the MO diagrams that show the orbital energies. N2+ has less bond energy.
What Is The Hybridization Of The Nitrogen Atoms In N2 ... Molecular Orbital Diagram of N2. Molecular orbitals exist in molecules where each molecule has its electron configuration in terms of a sigma bond and pi bond. According to molecular orbital theory, it tells about magnetic nature, stability order, and the number of bonds in a molecule.
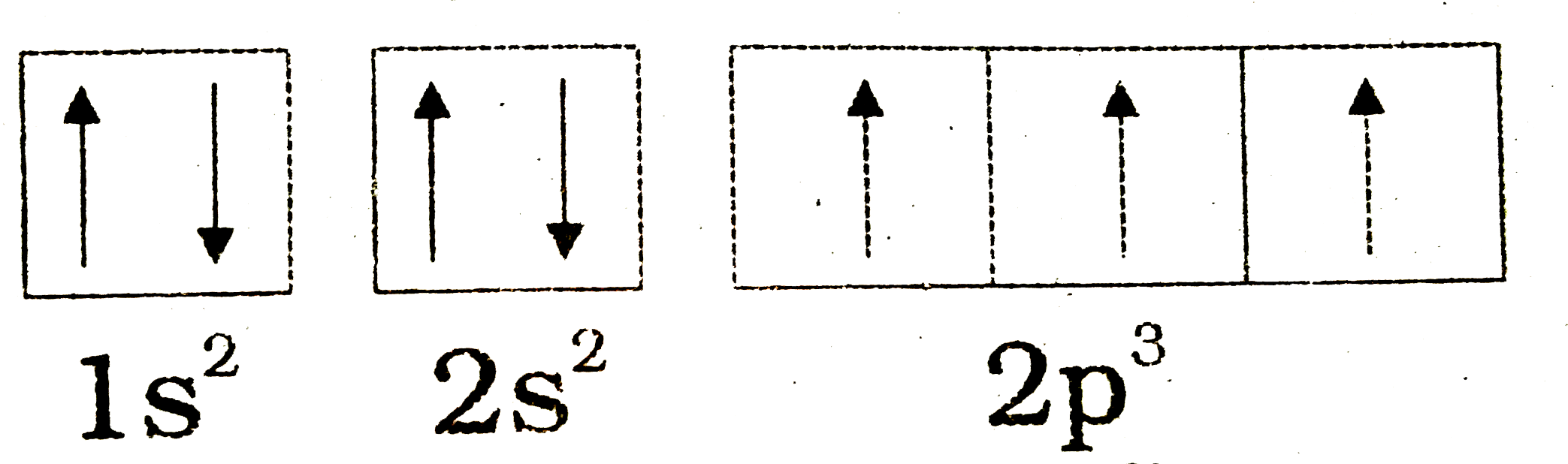
Orbital diagram of nitrogen
iperiodictable.com › nitrogen-electron-configurationOrbital Diagram For Nitrogen (N) | Nitrogen Electron ... Feb 15, 2021 · If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital. MOLECULAR ORBITAL DIAGRAM OF NITROGEN MOLECULE - YouTube In this vedio I explained Molecular Orbital Diagram of the Nnitogen molecule it's electronic configuration stability order magnetic character from the topic... Nitric Oxide Molecular Orbital Diagram Note that in this diagram the oxygen atomic orbitals are lower in energy than the nitrogen.Jul 21, · A molecular orbital diagram that can be applied to any homonuclear diatomic molecule with two identical alkali metal atoms Nitric oxide (NO) is an example of a heteronuclear diatomic molecule.
Orbital diagram of nitrogen. The orbital diagram for ground state nitrogen atom is: 1s ... The orbital diagram for ground state nitrogen atom is: 1s 2s 2p 1 L1l B 1 , 1L I_ c L L11 · Answer · Discussion · Video Transcript.4 answers · Top answer: So the first step is to go to the periodic table and signed the elements that we're looking ... schematron.org › orbital-filling-diagram-forOrbital Filling Diagram For Nitrogen - schematron.org Feb 09, 2018 · Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Nitrogen is the 7th element so the electron configuration number should add up to 7. 1s level can hold two electrons; 2s holds two; 2p holds six. are filled, we have to introduce a symbolic notation that scientists use to show orbital filling. Solved What is the orbital diagram for the valence ... What is the orbital diagram for the valence electrons in a ground state atom of nitrogen? Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Ans :- The orbital dia …. Orbital filling diagrams - The Cavalcade o' Chemistry The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ...
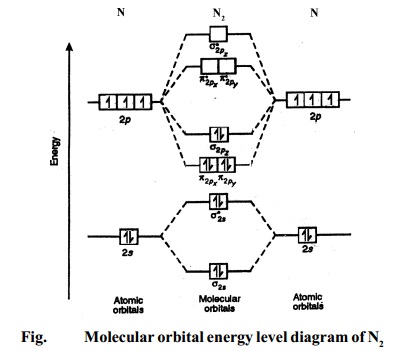
Draw the molecular orbital diagram for nitrogen gas ... Molecular orbital diagram of nitrogen gas is shown in the image. Since there are 7 electrons present in one nitrogen atom, so 14 electrons are... valenceelectrons.com › nitrogen-electron-configurationNitrogen(N) electron configuration and orbital diagram Nitrogen(N) is the 7th element in the periodic table and its symbol is 'N'. This article gives an idea about the electron configuration of nitrogen and orbital diagram, period and groups, valency and valence electrons of nitrogen, bond formation, compound formation, application of different principles.Hopefully, after reading this article you will know in detail about this. How to Do Orbital Diagrams - Sciencing Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. MO diagram for formation of nitrogen molecule from atoms ... MO diagram for formation of nitrogen molecule from atoms - Atomic and Molecular Orbitals The work of Chemissian molecular orbital editor is shown. Chemissian provides ability to work with several molecular orbital (and Kohn-Sham) diagrams at the same time.
Orbital Filling Diagram For Nitrogen - wiringall.com Orbital filling diagrams essentially just turn this big list of electron locations . In the same way, the orbital filling diagram for nitrogen will be. Nitrogen is the seventh element with a total of 7 electrons. In writing the electron configuration for nitrogen the first two electrons will go in the 1s orbital. Since 1s. Above orbital diagram shows the electron configuration of ... Above orbital diagram shows the electron configuration of nitrogen atom. Which rule does not support this? A. Afbau Principle. B. Hund's rule. C. Pauli's exclusion law. D. none of these. Medium. Open in App. Solution. Verified by Toppr. Correct option is B) What is the atomic orbital diagram for nitrogen? | Study.com These are also known as electron-in-a-box diagrams. This is a simplified diagram of how the electrons are arranged within the orbitals for a particular atomic ...1 answer · Top answer: Nitrogen From the periodic table, we can find Nitrogen which has an atomic number of 7. According to the Aufbau priniciple we have to completely... topblogtenz.com › nitrogen-orbital-diagramNitrogen Orbital diagram, Electron configuration, and Valence ... Orbital diagram for Nitrogen. The orbital diagram simply represents the arrangement of electrons in the different orbitals of an atom, it uses an arrow to represent the electrons, every orbital(one box) contains a maximum of 2 electrons. There are three rules followed for constructing the orbital diagram for an atom. (1).
write the molecular orbital diagram of n2 and calculate ... The molecular orbital diagram (MO Diagram) of N 2 + ion is ... why nitrogen have different structure of molecular orbital theory An atomic orbital is monocentric while a molecular orbital is polycentric. Explain What is the relationship between bond order and the dissociation energy of a molecule? ...
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Molecular orbitals in Nitrogen - ChemTube3D Home / Structure and Bonding / Atomic Orbitals / Molecular orbitals in Nitrogen CONTROLS Use the buttons to display the 1s and 2p atomic orbitals that make up the molecular orbitals.
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13 ...
Draw The Orbital Diagram Of The Element Nitrogen - hyundai Draw The Orbital Diagram Of The Element Nitrogen Draw The Orbital Diagram Of The Element Nitrogen - All over the eighties, Hyundai observed rapid advancement, producing substantial inroads into international markets. Having said that, until 1986, the corporation accomplished considered one of its key objectives: breaking in the American market.
Molecular Orbital Diagram of Nitrogen Molecule - Nature of ... Molecular Orbital Diagram of Nitrogen Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET...
Molecular orbital energy level diagrams -Hydrogen ... Molecular orbital energy level diagrams -Hydrogen, Hypothetical, Nitrogen, Oxygen. The filling of molecular orbitals is governed by the following principles. (i)Aufbau principle (ii)Pauli's exclusion principle and (iii)Hund's rule of maximum multiplicity. Now, let us consider some examples of homo nuclear diatomic molecules.
Draw the molecular orbital diagram of N2N2 + N2 Write ... Electrons of nitrogen are to be filled in this diagram. Left side represents the configuration of one atom of nitrogen molecule and the right side represents the second atom of nitrogen molecule. Atomic number of nitrogen is seven. Therefore in ${N_2}$ there are a total fourteen electrons. Molecular orbital diagram of ${N_2}$ is shown below:
Orbital Diagram For Nitrogen - Free PDF eBook Orbital Diagram For Nitrogen Free PDF eBooks. Posted on July 30, 2015. MO Diagrams for O2 and N2 - U of L Class Index. Drawing MO diagrams - always same number of MOs as AOs. ... N2 σ2s σ*2s σ* 2p π*2p π2p σ2p smaller ΔE. N2. Diamagnetic. Bond order of 3 (N≡N). Transition Metal Review.pdf.
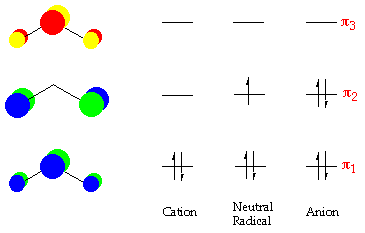
Draw the diagrams for "NO"_2^-, "NO"_2^+, and "NO"_2. The ... You've seen the molecular orbital (MO) diagram of "CO"_2: "CO"_2 and "NO"_2^+ are isoelectronic and thus have the same electron configuration. Thus, simply add one or two electrons into the 2b_(3u) and 2b_(2u) to get "NO"_2 and "NO"_2^-, respectively. Nitrogen atom has 2p atomic orbitals lower by "2.52 eV", and 2s atomic orbitals lower by "6.13 eV" than with carbon atom.
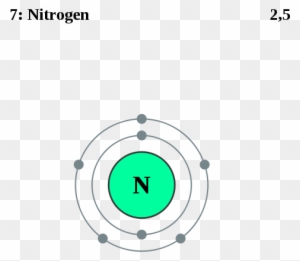
⚗️Which orbital diagram represents nitrogen (atomic number ... The choice A accurately specifies and illustrates the orbital diagram of a Nitrogen atom with 7 electrons. Based on the number of electrons in a Nitrogen atom, there are two energy levels, the s and p sub-levels: Nitrogen = 2, 5 . The first energy level, S will take up two electrons with opposite spin.
What is the orbital diagram for a ground-state nitrogen ... A nitrogen atom has 3 orbitals; the 1s orbital, the 2s orbital, and the 2p orbital. In this case, the 2s and 2p orbitals are the valence orbitals, as they have the electrons with the most energy.
Nitric Oxide Molecular Orbital Diagram Note that in this diagram the oxygen atomic orbitals are lower in energy than the nitrogen.Jul 21, · A molecular orbital diagram that can be applied to any homonuclear diatomic molecule with two identical alkali metal atoms Nitric oxide (NO) is an example of a heteronuclear diatomic molecule.
MOLECULAR ORBITAL DIAGRAM OF NITROGEN MOLECULE - YouTube In this vedio I explained Molecular Orbital Diagram of the Nnitogen molecule it's electronic configuration stability order magnetic character from the topic...
iperiodictable.com › nitrogen-electron-configurationOrbital Diagram For Nitrogen (N) | Nitrogen Electron ... Feb 15, 2021 · If you are still not getting the Nitrogen Electron Configuration of the element nitrogen then, the full electronic configuration of nitrogen is written as the following; 1s 2 2s 2 2p 3. If we gave you brief information then, the first two electrons lie in the 1s orbital, following the next 2 electrons, it comes under the 2s orbital.







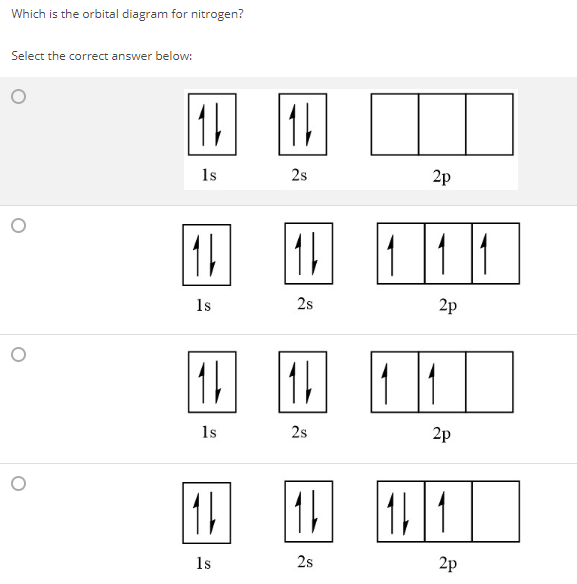

















0 Response to "41 orbital diagram of nitrogen"
Post a Comment