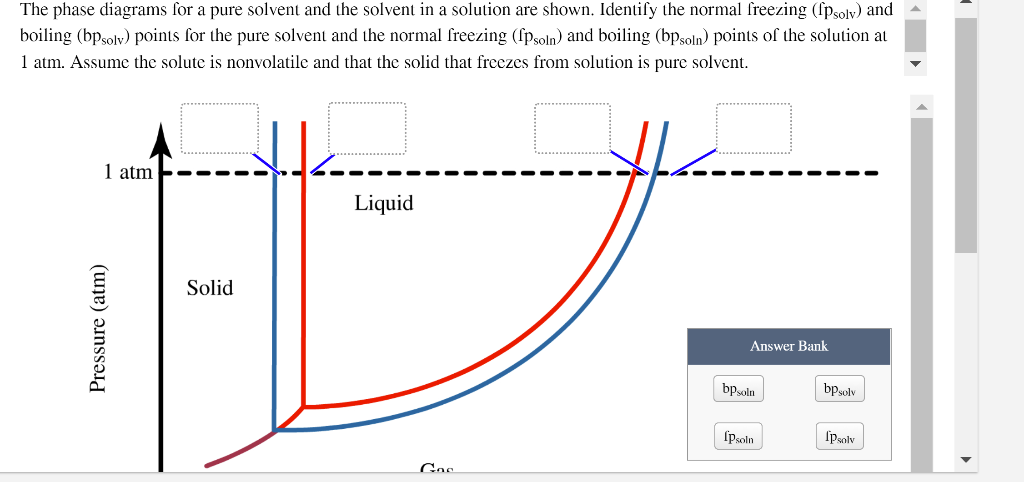
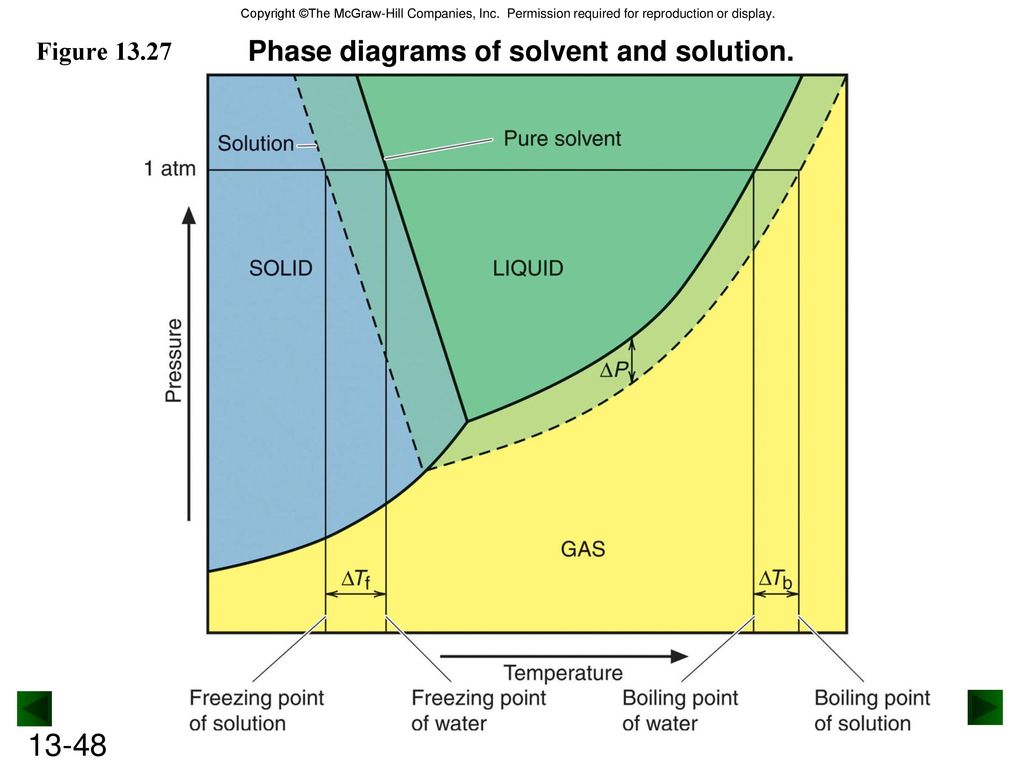
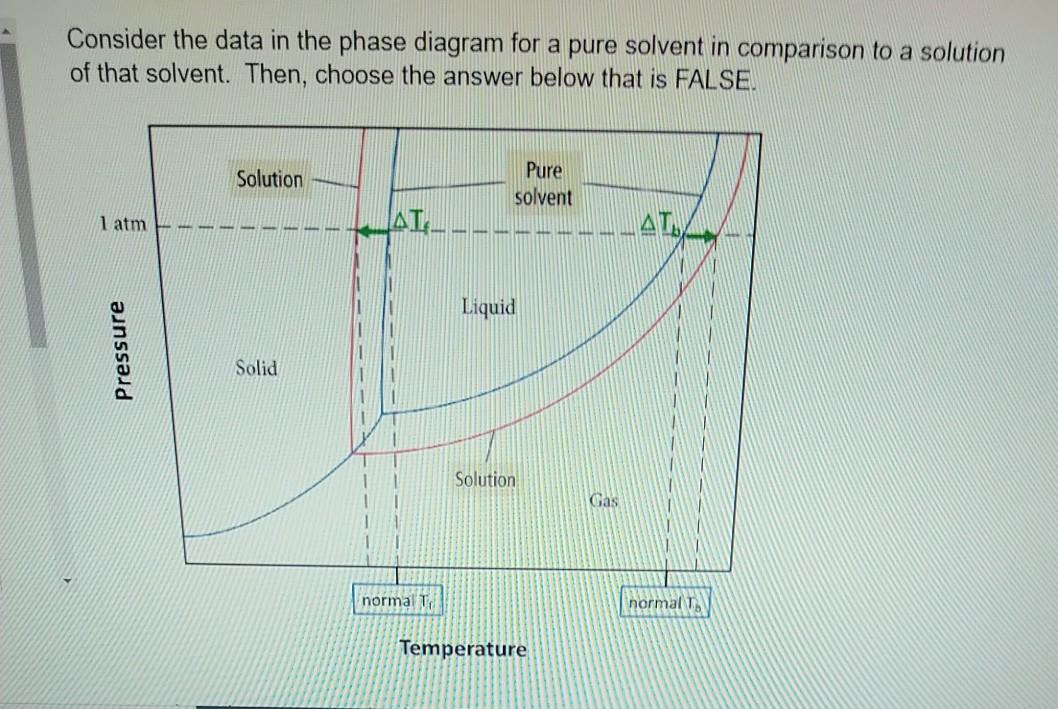
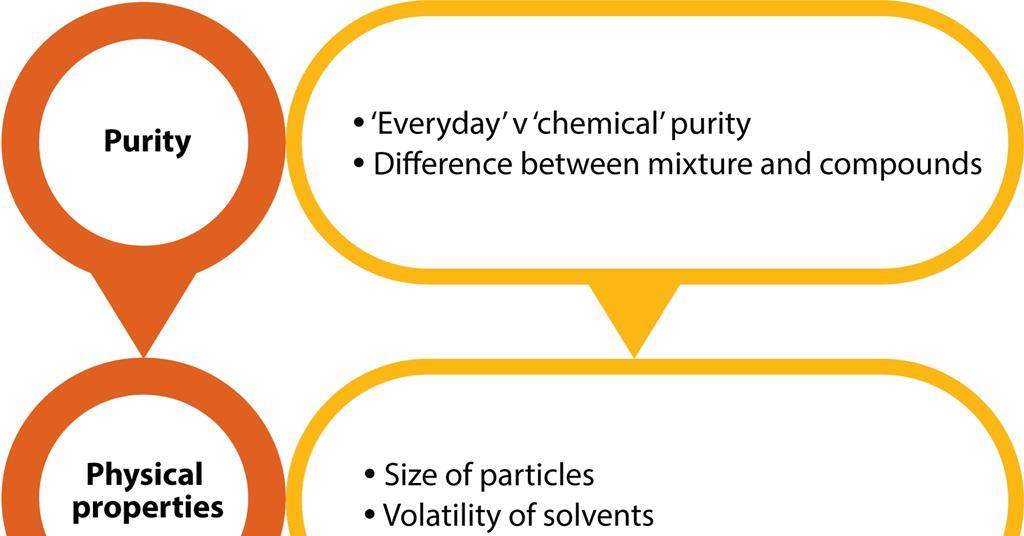
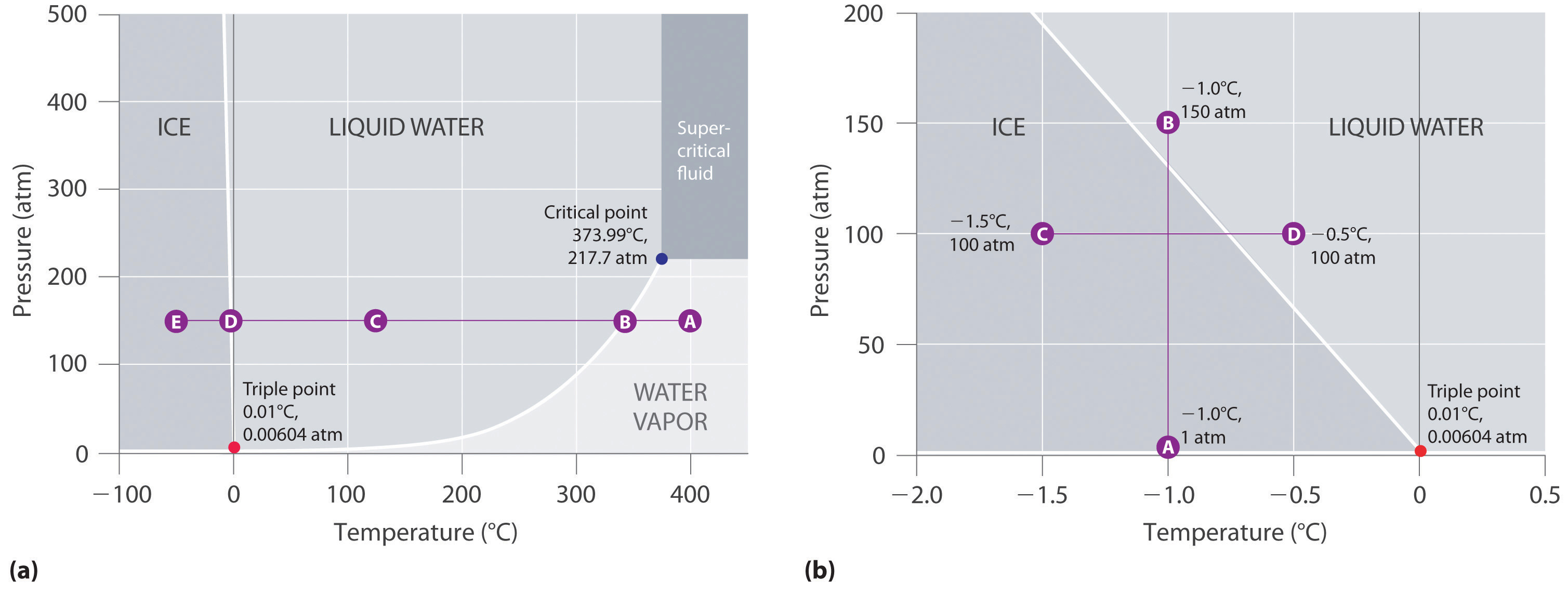
41 label the phase diagram of pure solvent and a solution
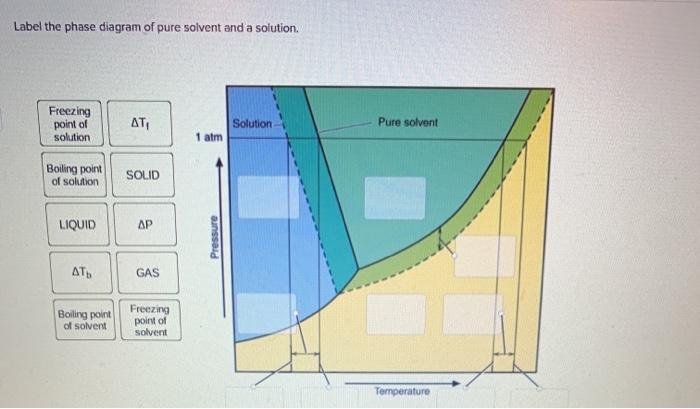
The figure shows two phase diagrams, one for a pure liquid ... Consider the following general phase diagram: Now, consider the pure substance, whose phase diagram was represented by the black curves. The normal freezing point of the pure substance at constant pressure is indicated by B, and its reduced normal freezing point (due to addition of nonvolatile solute to the pure liquid substance) is A, since Tdarr leftwards. Solved Label the phase diagram of pure solvent and a ... We review their content and use your feedback to keep the quality high. 100% (10 ratings) Transcribed image text: Label the phase diagram of pure solvent and a solution Freezing point of solution GAS Solution Pure solvent 1 atm Boiling pointFreezing point of of solvent solvent ??? 11 SOLID AT AP Boiling pointLIQUID of solution Temperature.
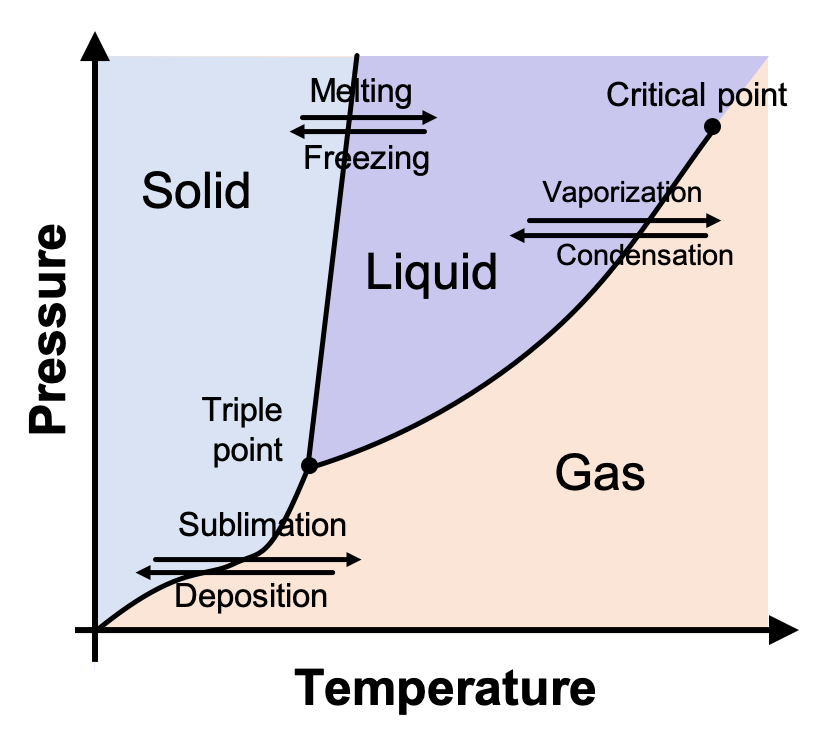
10.4 Phase Diagrams – Chemistry - BC Open Textbooks A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2.
Label the phase diagram of pure solvent and a solution
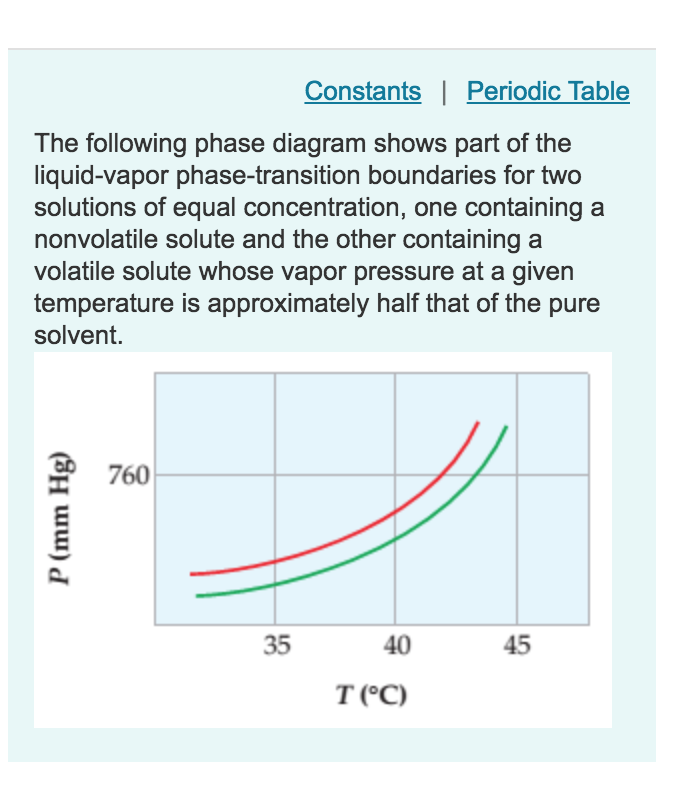
PDF 5.8 Liquid-Liquid phase diagrams (a) Eutectics • a 1 → a 2: The system enters the two-phase region labeled 'Liquid+B'. Pure solution begins to come out of solution and remaining liquid becomes richer in A • a 2 → a 3: More of the solid B forms • a 3 → a 4: At the end of this step, there is less liquid a Label the phase diagram of a pure solvent and a solution. Dec 11, 2019 — Label the phase diagram of a pure solvent and a solution. Answer. +20. Watch. 1. answer. 0. watching. 53. views. Freezing Point Depression | Chemistry for Non-Majors Freezing Point Depression. The figure below shows the phase diagram for a pure solvent and how it changes when a solute is added to it.The solute lowers the vapor pressure of the solvent resulting in a lowering of the freezing point of the solution compared to the solvent.
Label the phase diagram of pure solvent and a solution. Label the phase diagram of pure solvent and a solution ... Feb 24, 2022 · Label the phase diagram of pure solvent and a solution. Freezing point of solution ΔΤ, Solution Pure solvent 1 atm Boiling point of solution SOLID LIQUID AP Pressure AT) GAS Boiling point of solvent Freezing point of solvent Temperature Phase Diagram Labels - 18 images - label the phase diagram of ... 31 label the phase diagram of pure solvent and a solution. Phase Diagram Labels. Here are a number of highest rated Phase Diagram Labels pictures on internet. We identified it from honorable source. Its submitted by dealing out in the best field. We allow this kind of Phase Diagram Labels graphic could possibly be the most trending subject ... Chemistry 1 Exam Flashcards - Quizlet Identify the solvent and solute in each of the solutions A) salt (NaCl) in water ... Also, assume that the engine coolant is pure ethylene glycol (HOCH2CH2OH), which is non-ionizing and non-volatile, and that the pressure remains constant at 1.00 atm. ... Label the phase diagram. A) Solid Phase B) Triple Point C) Sublimation Point/ Deposition Point Phase Diagrams | Boundless Chemistry - Lumen Learning A phase diagram is a graph which shows under what conditions of temperature and pressure distinct phases of matter occur. The simplest phase diagrams are of pure substances. These diagrams plot pressure on the y-axis and temperature on the x-axis. Although phases are conceptually simple, they are difficult to define precisely.
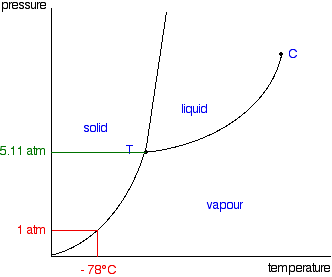
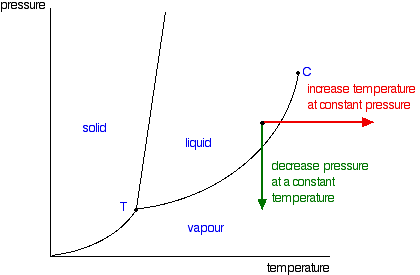
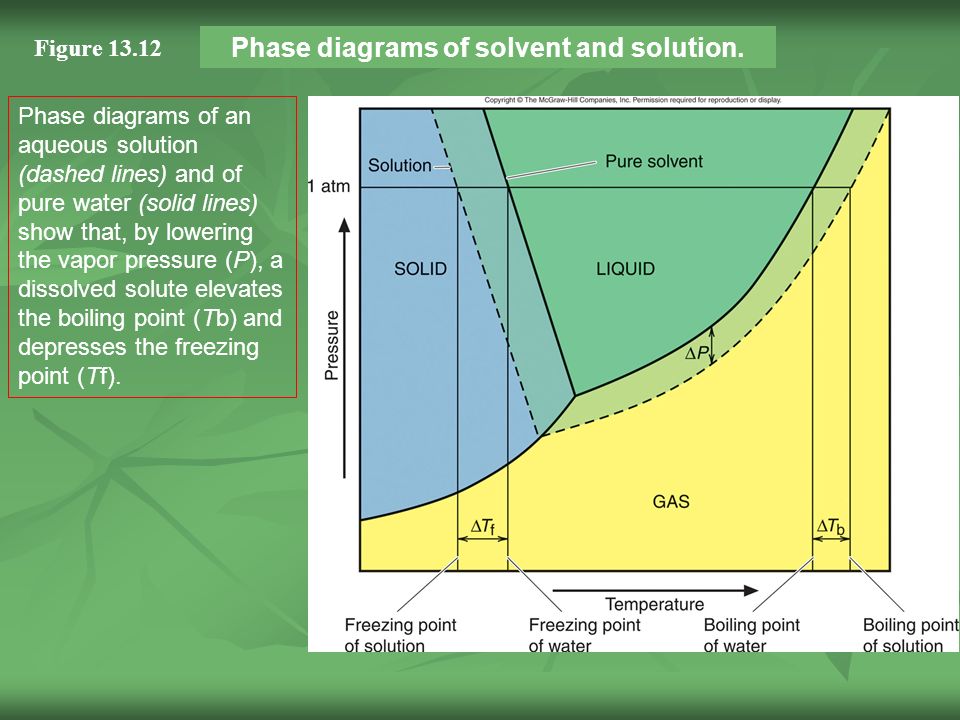
phase diagram solvent solution - Just Only Pure solvent Freezing point of solution Freezing point of water ... Boiling point of solution 1 atm Pressure ΔTf ΔTb ΔP Temperature. Title: phase_diagram_solvent ... PDF Phase Diagrams, Solid Solutions, Phase Transformations Isomorphous Phase Diagrams Phase diagram Indicate phases as a function of Temp., Comp. and Pressure (under equilibrium condition) Binary phase diagram A phase diagram for a system with two components. Isomorphous phase diagram A phase diagram in which the components display unlimited solid solubility. Chapter 10: Solid Solutions and Phase ... phase diagrams of pure substances - chemguide Phase diagrams. A phase diagram lets you work out exactly what phases are present at any given temperature and pressure. In the cases we'll be looking at on this page, the phases will simply be the solid, liquid or vapour (gas) states of a pure substance. This is the phase diagram for a typical pure substance. The phase diagram for solvent and solutions is shown in the ... >> The phase diagram for solvent and soluti. Question . The phase diagram for solvent and solutions is shown in the figure. What represents the normal boiling point of the solution? A. A. B. B. C. C. D. D. Hard. Open in App. Solution. Verified by Toppr. ... The phase diagram for a pure substance is shown above.
Solved Label the phase diagram of pure solvent and a | Chegg.com Chemistry. Chemistry questions and answers. Label the phase diagram of pure solvent and a solution. Freezing point of solution ΔΤ, Solution Pure solvent 1 atm Boiling point of solution SOLID LIQUID AP Pressure AT) GAS Boiling point of solvent Freezing point of solvent Temperature. Phase Diagrams for Pure Substances - Chemistry LibreTexts Aug 15, 2020 — The critical point. You will have noticed that this liquid-vapor equilibrium curve has a top limit (labeled as C in the phase diagram), which is ... PDF Chapter 9: Phase Diagrams - Florida International University - Solutions - solid solutions, single phase - Mixtures - more than one phase • Solubility Limit : Max concentration for which only a single phase solution occurs. Question: What is the solubility limit at 20°C? Answer: 65 wt% sugar . If Co < 65 wt% sugar: syrup If Co > 65 wt% sugar: syrup + sugar. 65 Sucrose/Water Phase Diagram Pure ... Label the diagram of pure solvent and a so... | Clutch Prep Problem: Label the diagram of pure solvent and a solution. FREE Expert Solution Recall that a phase diagram shows the transition of matter between solid, liquid, and gas phases as temperature and pressure changes.
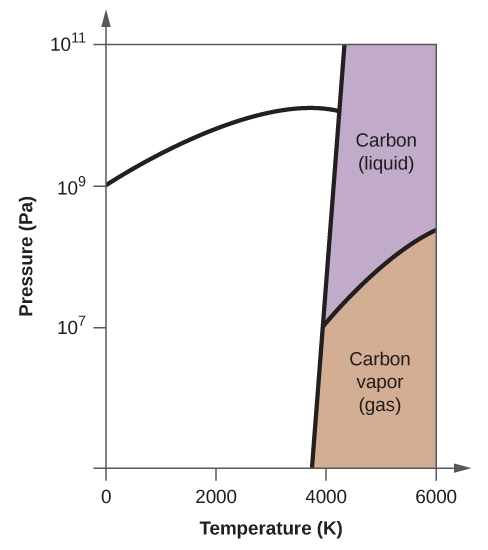
Phase Diagrams - Chemistry Elemental carbon has one gas phase, one liquid phase, and two different solid phases, as shown in the phase diagram: (a) On the phase diagram, label the gas and liquid regions. (b) Graphite is the most stable phase of carbon at normal conditions. On the phase diagram, label the graphite phase.
PDF Phase Diagrams Phase Diagrams Revised: 1/27/16 3 The phase diagram in Figure 1 is for a pure compound. When a second compound is introduced to the system forming a homogeneous solution however, the phase diagram drastically changes. For example, the addition of a solute to a pure solvent (making a solution)
Label the phase diagram of pure solvent and a solution ... Label the phase diagram of pure solvent and a solution. Question: Label the phase diagram of pure solvent and a solution. This problem has been solved! See the answer See the answer See the answer done loading. Show transcribed image text Expert Answer. Who are the experts?
Freezing Point Depression | Chemistry for Non-Majors Freezing Point Depression. The figure below shows the phase diagram for a pure solvent and how it changes when a solute is added to it.The solute lowers the vapor pressure of the solvent resulting in a lowering of the freezing point of the solution compared to the solvent.
Label the phase diagram of a pure solvent and a solution. Dec 11, 2019 — Label the phase diagram of a pure solvent and a solution. Answer. +20. Watch. 1. answer. 0. watching. 53. views.
PDF 5.8 Liquid-Liquid phase diagrams (a) Eutectics • a 1 → a 2: The system enters the two-phase region labeled 'Liquid+B'. Pure solution begins to come out of solution and remaining liquid becomes richer in A • a 2 → a 3: More of the solid B forms • a 3 → a 4: At the end of this step, there is less liquid a
















0 Response to "41 label the phase diagram of pure solvent and a solution"
Post a Comment