42 orbital diagram for rubidium
Rubidium (Rb) has an atomic mass of 37. ... Electron Configuration, [Kr] 5s1. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 5s1. Orbital Diagram.Atomic Number: 37Atomic Weight: 85.4678 Isotopes Jan 01, 2022 · Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram of ...
... Physical Properties, Thermal Properties, Crystal Structure, Atomic & Orbital Properties, electron configuration, Chemical Properties rubidium, Isotopes, ...
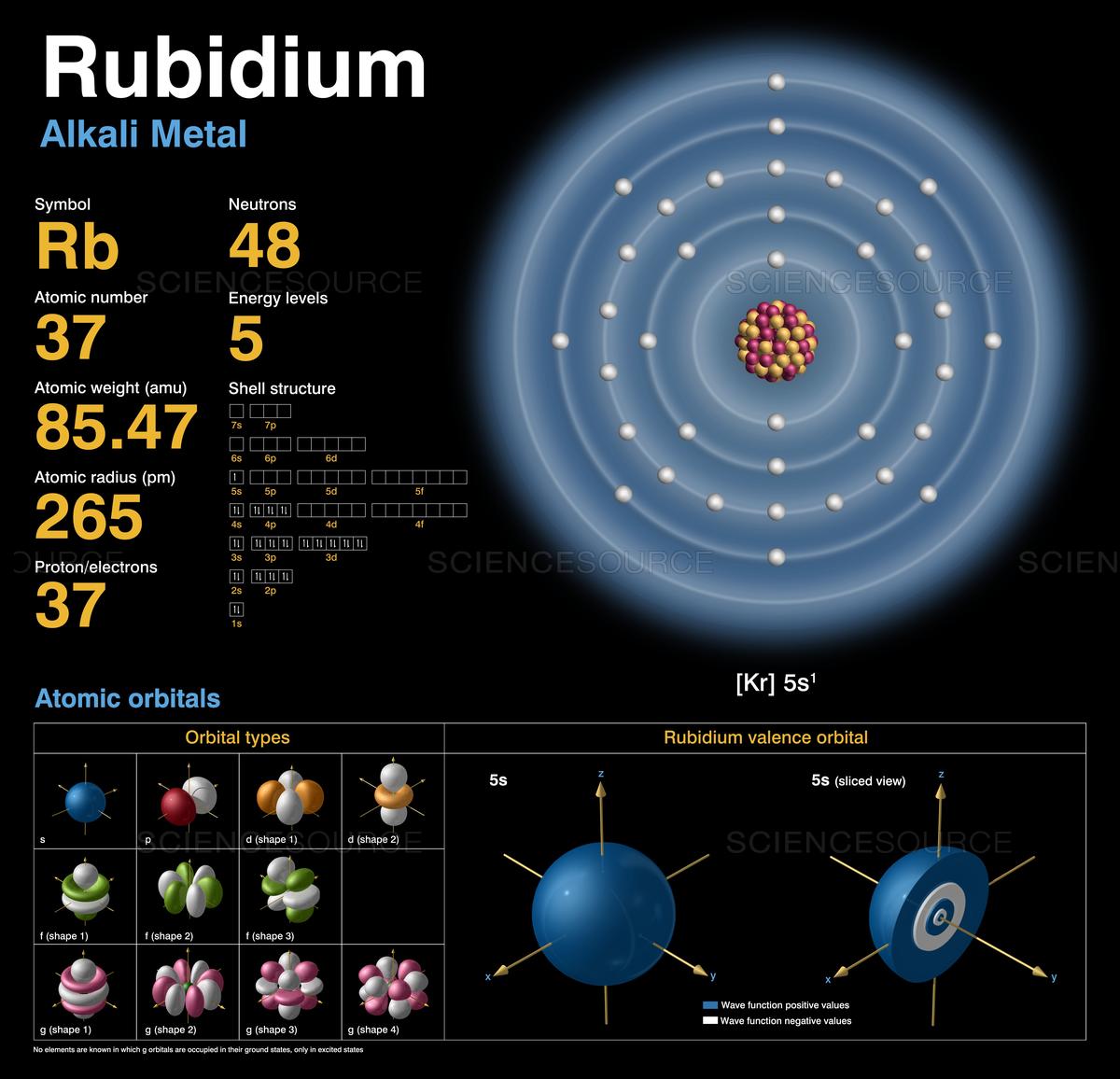
Orbital diagram for rubidium
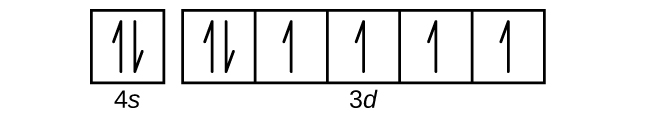
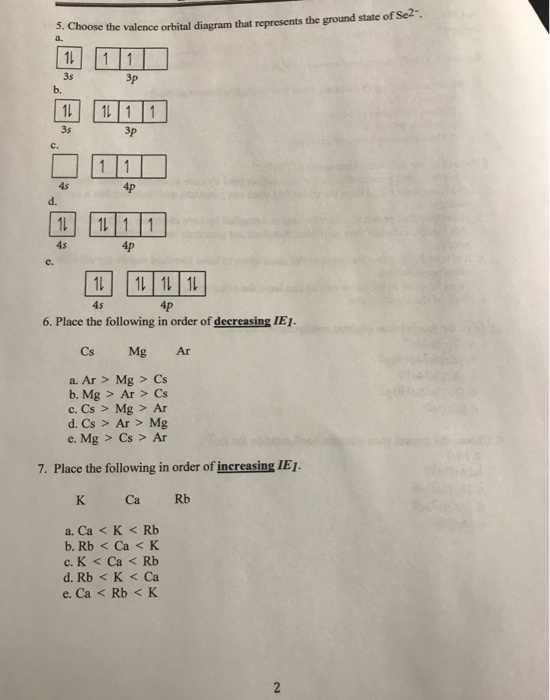
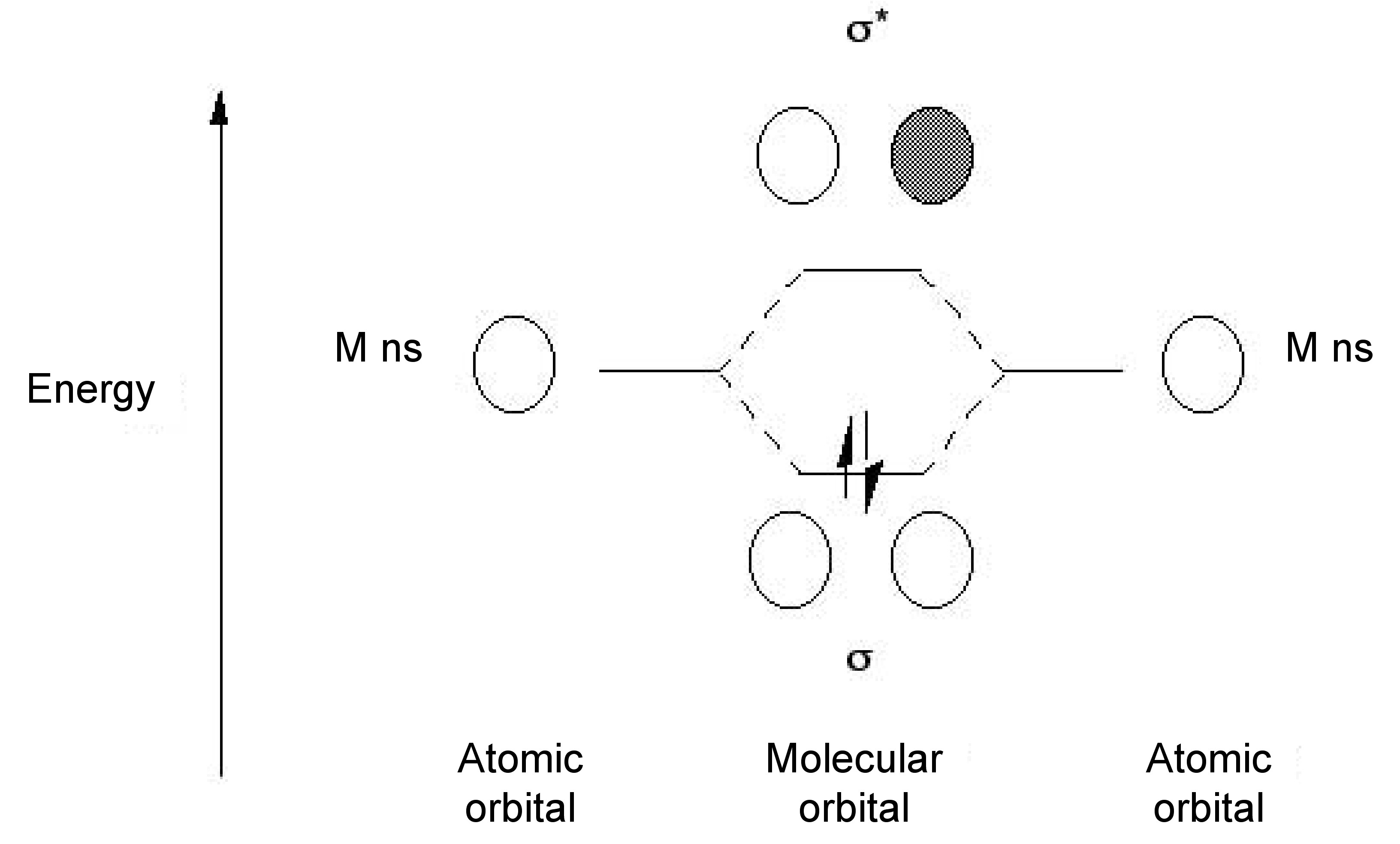
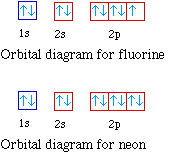
Sep 15, 2018 · The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium. Answer to: Write the electron configuration, orbital filling diagram, and determine the number of valence electrons for the Rb. What is similar to...1 answer · Top answer: Rubidium has 37 electrons so its electronic configuration is equal to 1s22s22p63s23p64s23d104p65s11s22s22p63s23p64s23d104p65s1 and its... Jan 18, 2021 · Rubidium Atomic and Orbital Properties Rubidium atoms have 37 electrons and the electronic shell structure is [2, 8, 18, 8, 1] with Atomic Term Symbol (Quantum Numbers) 2S1/2. What is the order of orbital diagram?
Orbital diagram for rubidium. ... the electron configuration and give the orbital diagram of a rubidium (Rb) ... in writing down the electron configuration for the specified element. Aug 02, 2019 · Orbital Diagram For Rubidium 08.02.2019 08.02.2019 3 Comments on Orbital Diagram For Rubidium Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of rubidium (atomic number: 37), the most. partial orbital diagram: 4s: UP/DOWN 3d1: UP/DOWN 3d2: UP/DOWN 3d3: UP 3d4: UP 3d5: UP (b) Rb (Z = 37 ... Rubidium atoms have 37 electrons and the shell structure is 2.8.18.8.1. The ground state electron configuration of ground state gaseous neutral rubidium is [Kr] ... 14 Feb 2021 — The electron configuration of Rubidium can be written as [Kr] 5s1 and here is the simple explanation behind this configuration. We know that the ...
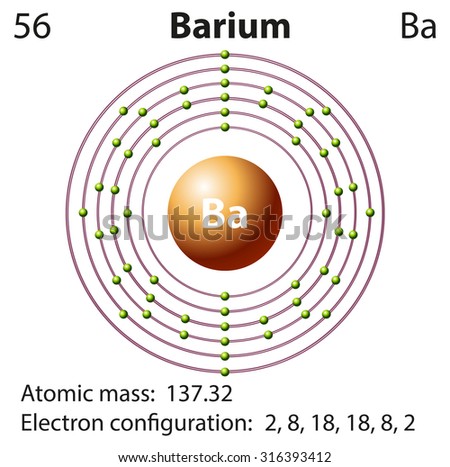
Jun 01, 2012 · Rubidium has an atomic number of 37, making it an alkali metal. This means that its last shell is an s with only one electron. The full notation is [Kr] 5s1. Jan 18, 2021 · Rubidium Atomic and Orbital Properties Rubidium atoms have 37 electrons and the electronic shell structure is [2, 8, 18, 8, 1] with Atomic Term Symbol (Quantum Numbers) 2S1/2. What is the order of orbital diagram? Answer to: Write the electron configuration, orbital filling diagram, and determine the number of valence electrons for the Rb. What is similar to...1 answer · Top answer: Rubidium has 37 electrons so its electronic configuration is equal to 1s22s22p63s23p64s23d104p65s11s22s22p63s23p64s23d104p65s1 and its... Sep 15, 2018 · The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of .. Rb+, Se2−. The first orbital (an s orbital) can contain only two electrons.. Rubidium.
















:max_bytes(150000):strip_icc()/Rubidium-58b601ee3df78cdcd83d20d6.jpg)
0 Response to "42 orbital diagram for rubidium"
Post a Comment