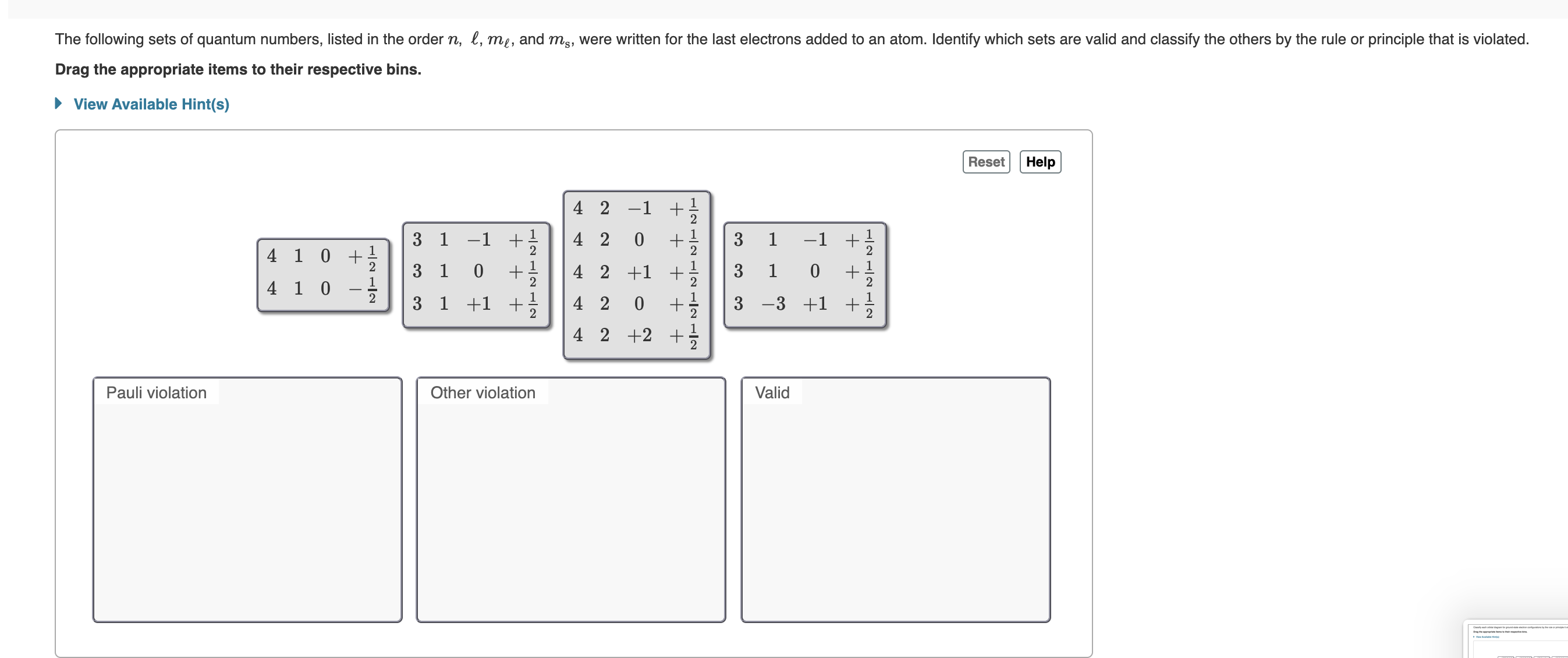
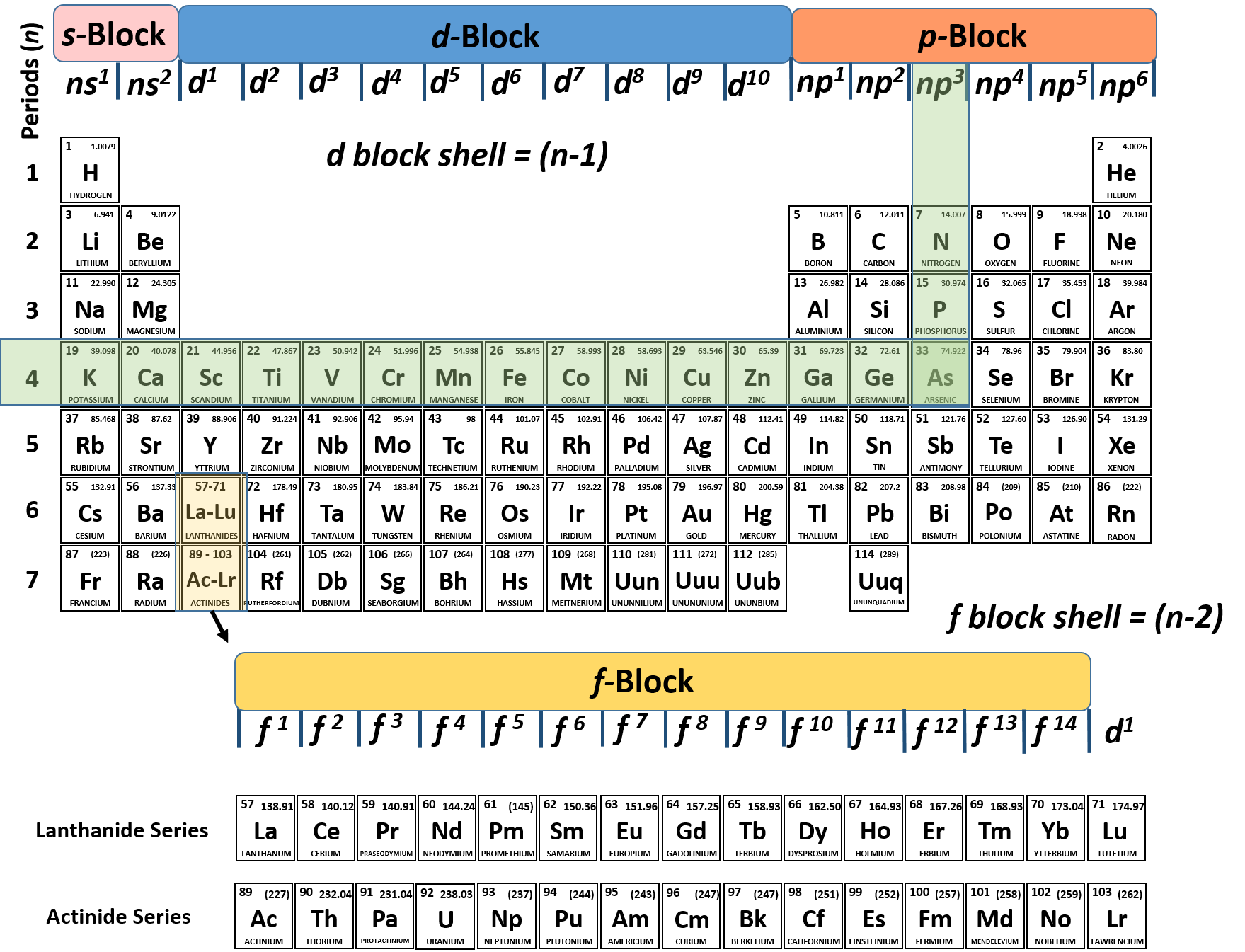
40 match the diagram below to the transition metal it represents.
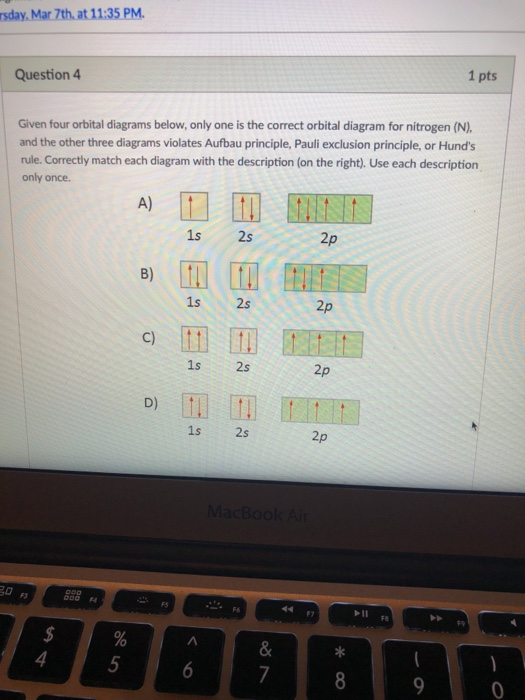
In a metal, the bottom half of the band is composed of bonding molecular orbitals and the ... Match the diagram below to the transition metal it represents. Match. Gravity. Created by. maddiejb13. Terms in this set (70) ... A student draws the orbital diagram below for the 3d electrons in a V atom. What, if anything, is incorrect about the drawing? ... Which of the following electron configurations represent a transition metal atom?
The atomic numbers and electronic configurations of the given elements are Element Atomic ... Match the diagram below to the transition metal it represents.

Match the diagram below to the transition metal it represents.
6. Which of the following compounds best fits the PMR spectrum shown below. The table shows the chemical shift for groups attached to a bromine atom or a cyano group measured to the center of the multiplet. 7. Which of the following alkyl groups would show the multiplet pattern depicted in the diagram? Match the diagram below to the transition metal it represents. Transition metals or inner transition metals. For the bulk metal up to 12 electrons per atom can occupy the composite band since the composite band is built up from five 3d orbitals and one 4s orbital. From wikibooks open books for an open world. The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. ... The transition state is the point of highest energy between the reactant(s) and product(s). ... such as the platinum metal added to the H 2 /O 2 mixture ...
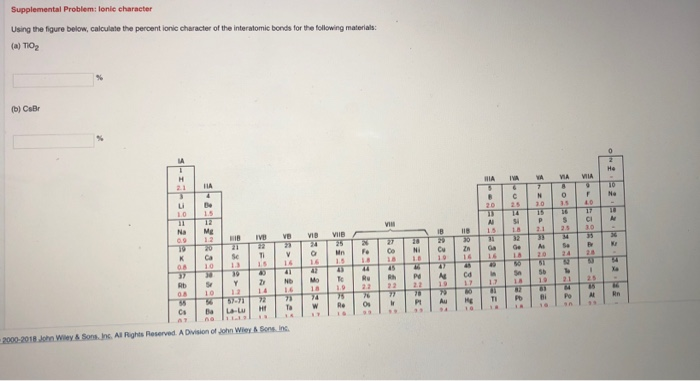
Match the diagram below to the transition metal it represents.. Coordination Complexes. Coordination compounds, such as the FeCl 4-ion and CrCl 3 6 NH 3, are called such because they contain ions or molecules linked, or coordinated, to a transition metal.They are also known as complex ions or coordination complexes because they are Lewis acid-base complexes. The ions or molecules that bind to transition-metal ions to form these complexes are called ligands ... In octahedral complexes, the molecular orbitals created by the coordination of metal center can be seen as resulting from the donation of two electrons by each of six σ-donor ligands to the d-orbitals on the metal. The metal orbitals taking part in this type of bonding are nd, (n+1)p and (n+1)s. It should be noted down The chart below shows the percentages of elements in the Earth's crust. Excluding the "Other" category, what percentage of the Earth's crust is . a. alkali metals? 5.4%. b. alkaline-earth metals? 5.6%. INTERPRETING GRAPHICS . 19. Study the diagram below to determine the pattern of the images. Predict the missing image, and draw it ... The _____ sphere is enclosed in brackets in formulas for complex species, and it includes the central metal ion plus the coordinated groups. (a) ligand (b) donor (c) oxidation (d) coordination (e) chelating 2. In coordination chemistry, the donor atom of a ligand is (a) a Lewis acid. (b) the counter ion (c) the central metal atom.
Metallic properties, including melting point, ductility, and conductivity, can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents. sorry i couldnt figure how to rotate it! Z represents O2 binding in cells with elevated 2,3-BPG, whereas X represents O2 binding in cells with decreased 2,3-BPG In one type of hemoglobin mutant the amino acid change generates a strong ionic interaction stabilizing the T state conformation but only under conditions of lower pH, e.g., at pH 7.2 compared to pH 7.6. Base your answers to questions 1 and 2 on the information below and on your knowledge of chemistry. The bright-line spectra observed in a spectroscope for three elements and a mixture of two of these elements are represented in the diagram below. Selected Bright-Line Spectra Element A Element D Element Z Mixture 700 600 500 400 Wavelength (nm) l. Transition metals from the 4th period have a band of molecular orbitals formed from a ... Match the diagram below to the transition metal it represents.1 answer · Top answer: The conductivity can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled with electrons.[readmore]We can ...
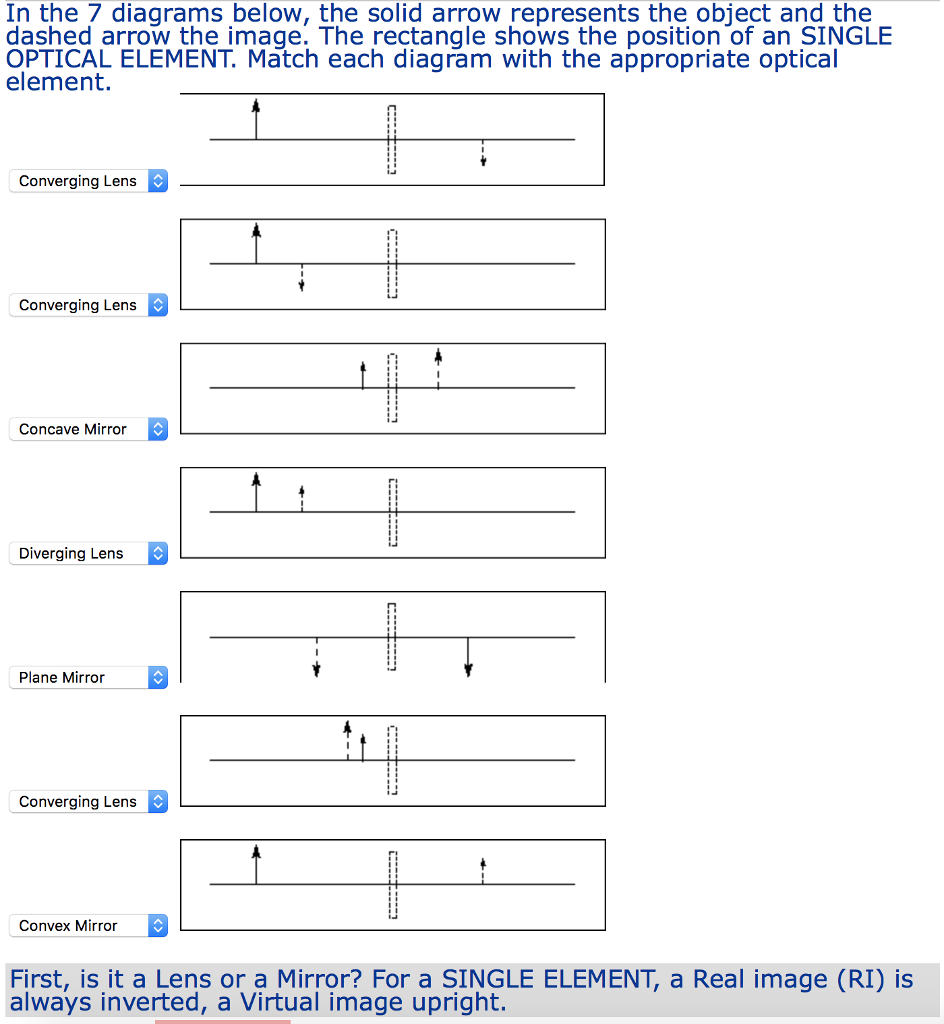
A typical phase diagram for a pure substance is shown in Figure 1. Figure 1. The physical state of a substance and its phase-transition temperatures are represented graphically in a phase diagram. To illustrate the utility of these plots, consider the phase diagram for water shown in Figure 2. Figure 2. A structural diagram of what this molecule would look like is shown below. Note that each straight line is being used here to indicate a covalent bond within the phosphate ion. Each straight line represents two electrons (or an electron pair) that is being shared between the atoms. Aug 13, 2020 — The characteristics of transition metal-ligand bonds become clear by ... of the σ and π molecular orbitals are described step by step below. 33.The diagram above represents the uniform heating of a substance that is a solid at t0. What is the freezing point of the substance? A)EF B)BC C)CD D)DE 34.The graph below represents the uniform cooling of a sample of a substance, starting with the substance as a gas above its boiling point. Which segment of the curve represents a time when
... by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents.
We see from the energy level diagram that the energy levels get closer together as #n# increases. This, the smallest energy and the longest wavelength is associated with the #n = 7 → n = 8# transition. I tried to mark it with an arrow in the diagram, but the lines are so close together that all you can see is the red triangle of the arrowhead.
• suggested in 1893 that metal ions have primary and secondary valences. ! Primary valence equals the metal’s oxidation number ! Secondary valence is the number of atoms directly bonded to the metal (coordination number) Co(III) oxidation state Coordination # is 6 Cl-
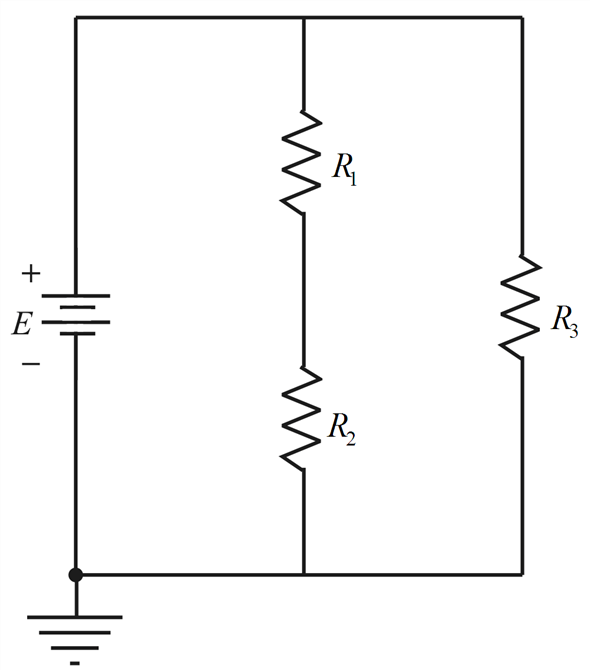
5. A 28.2 gram sample of a metal is heated to 99.8˚C and then placed in a coffee-cup calorimeter containing 150.0 grams of water at 23.5˚C. After the metal cools, the final temperature of the metal-water mixture is 25˚C. Calculate the specific heat of the metal, assuming no heat escapes to the surroundings or is transferred to the ...
Transition metals from the 4th period have a band of molecular orbitals formed from a ... Match the diagram below to the transition metal it represents.1 answer · Top answer: Solution :- following image shows the classification of the element with the band theory prediction of the energy and band gap
overlap of 2p orbitals is above and below, if in the plane of our paper, or in front and in back, if perpendicular to the plane of our paper. The picture of two interacting 2p orbitals looks something like the following. node = zero electron density because of opposite phases 2pa π bond LUMO HOMO π = 2pa + 2pb = bonding MO = potential energy ...
James C. Dabrowiak · 2009 · ScienceFor transition metal complexes which contain many atoms there are often more ... orbitals than any of the metal atomic orbitals in the diagram means that ...
There is, however, a noticeable difference in the transition state, which is distinctly lower in diagram (b) than it is in (a). This indicates the use of a catalyst in diagram (b). The activation energy is the difference between the energy of the starting reagents and the transition state—a maximum on the reaction coordinate diagram.
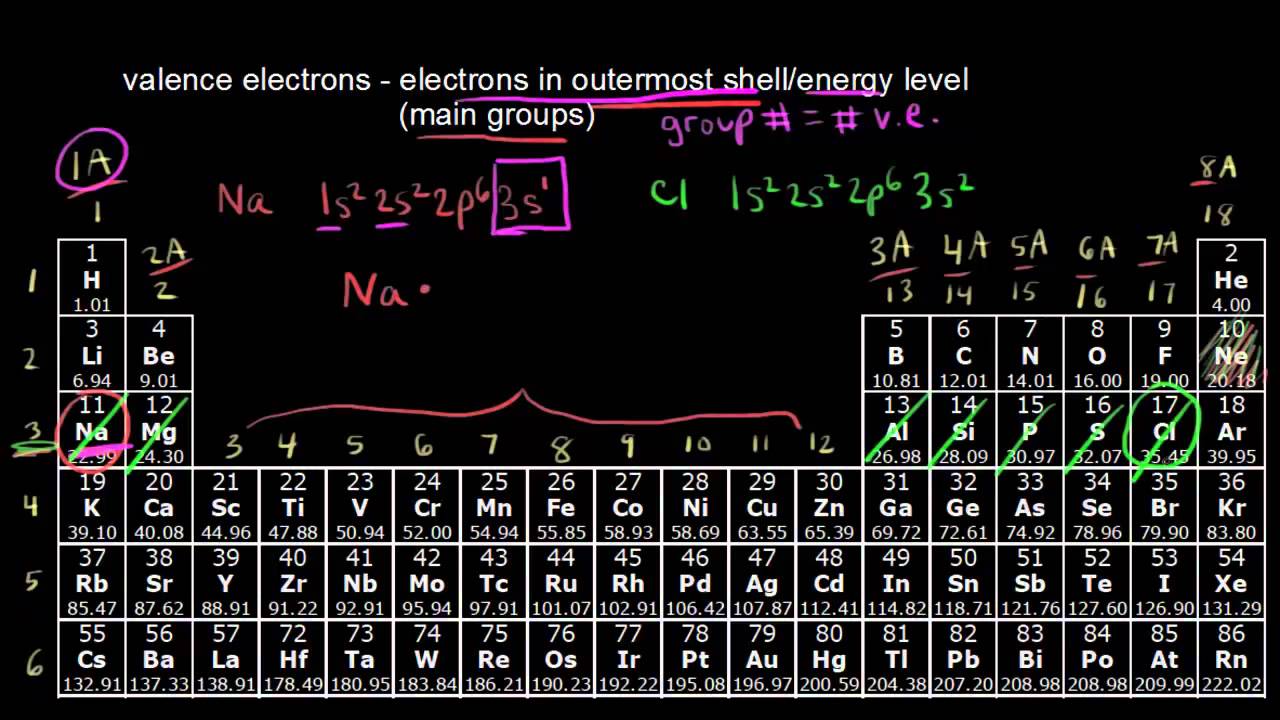
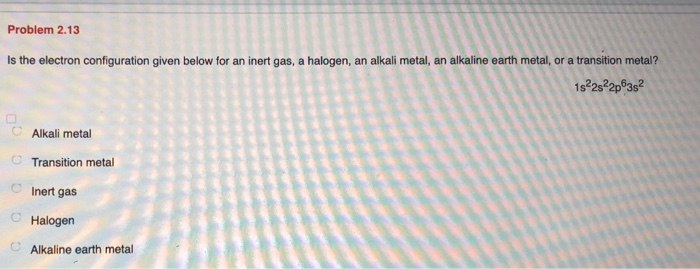
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 2p^5#
The diagram shown below is that for a medium-carbon structural steel. Metallic engineering materials are classified as either ductile or brittle materials. A ductile material is one having relatively large tensile strains up to the point of rupture like structural steel and aluminum, whereas brittle materials has a relatively small strain up to ...
Phase Diagrams • Indicate phases as function of T, Co, and P. • For this course:-binary systems: just 2 components.-independent variables: T and Co (P = 1 atm is almost always used). • Phase Diagram for Cu-Ni system Adapted from Fig. 9.3(a), Callister 7e. (Fig. 9.3(a) is adapted from Phase Diagrams of Binary Nickel Alloys , P. Nash
Metallic properties, including melting point, ductility, and conductivity, can be predicted by the degree to which the bonding and anti-bonding portions of the band are filled. Match the diagram below to the transition metal it represents.
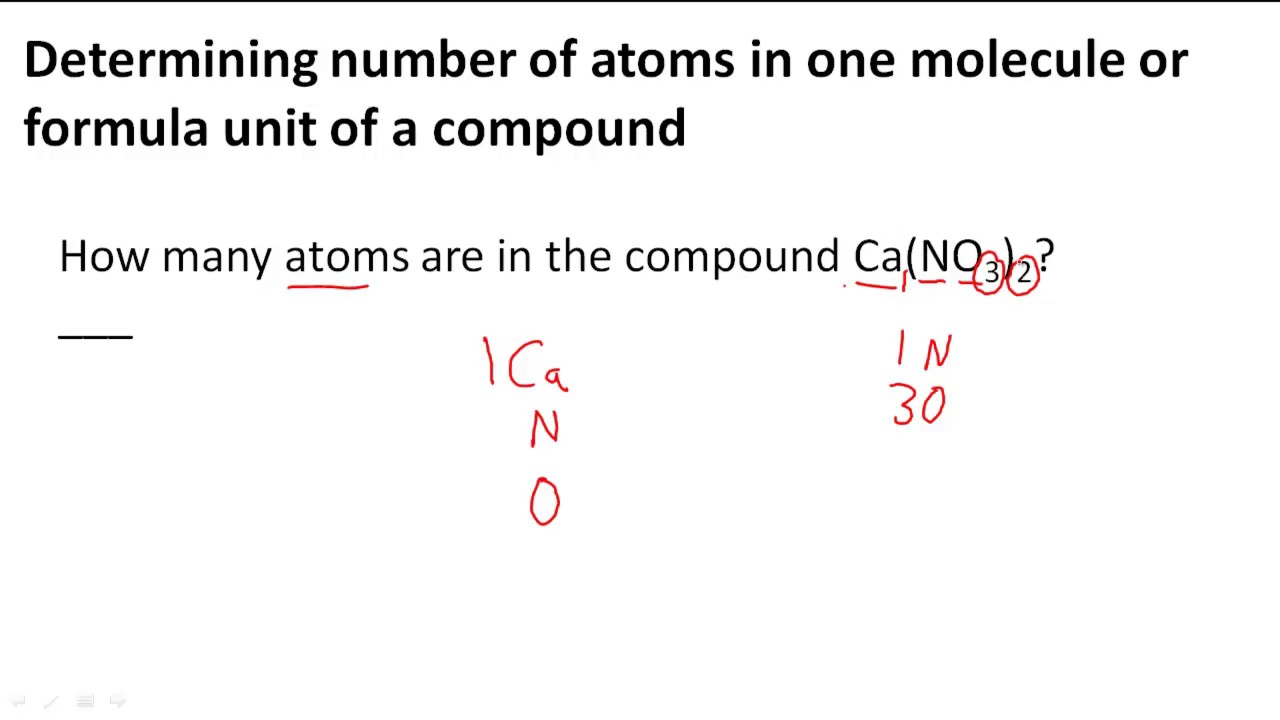
Lewis structures (also known as Lewis dot structures or electron dot structures) are diagrams that represent the valence electrons of atoms within a molecule. These Lewis symbols and Lewis structures help visualize the valence electrons of atoms and molecules, whether they exist as lone pairs or within bonds.
According to the Bohr model, the wavelength of the light emitted by a hydrogen atom when the electron falls from a high energy (n = 4) orbit into a lower energy (n = 2) orbit.Substituting the appropriate values of R H, n 1, and n 2 into the equation shown above gives the following result.. Solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental ...
This photo about: Match the Diagram Below to the Transition Metal It Represents., entitled as Electronic Spectroscopy Interpretation Chemistry Libretexts Match The Diagram Below To The Transition Metal It Represents. - also describes Electronic Spectroscopy Interpretation Chemistry LibreTexts and labeled as: ], with resolution 2758px x 1012px
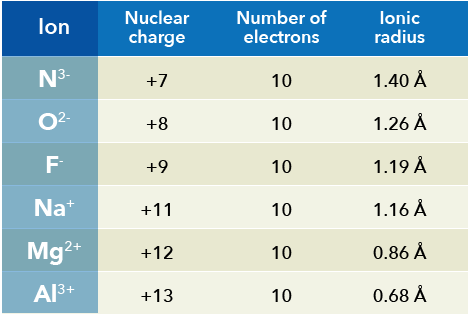
Yet, since Cr is a first row transition metal, the ionic radius is still small enough that the coordination sphere can not expand to 7-coordinate (or higher) without introducing sizable steric repulsions. These combination of factors (and probably others) lead to the high incidence of 6-coordinate Cr 3+ complexes.
The Aufbau principle predicts that the 4s orbital is always filled before the 3d orbitals, but this is actually not true for most elements! From Sc on, the 3d orbitals are actually lower in energy than the 4s orbital, which means that electrons enter the 3d orbitals first. In this video, we'll discuss this in more depth and walk through all of the electron configurations for the 3d ...
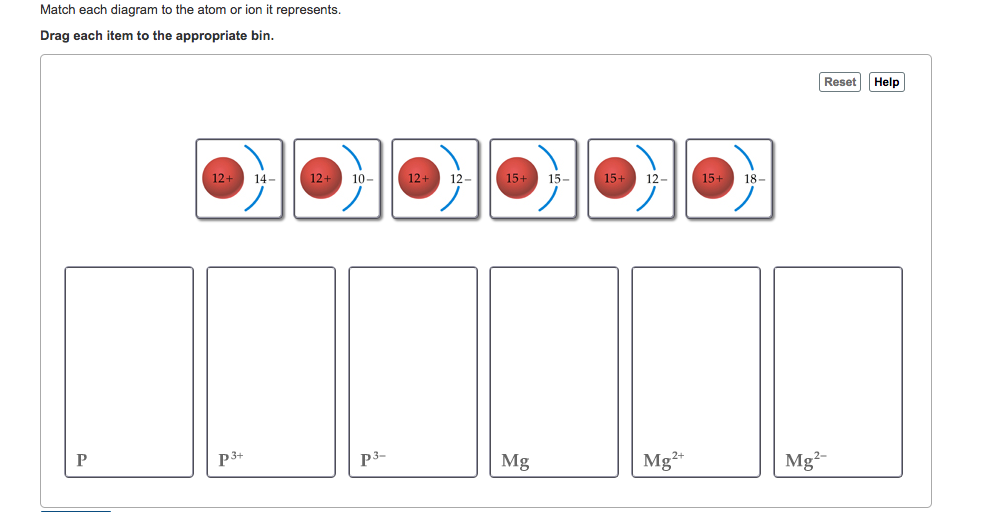
Match the Diagram Below to the Transition Metal It Represents. band theory match the diagram to the transition metal it match the diagram to the transition metal it represents what band represents the truest form of metal music which albums by metal mastering chemistry chapter 2 assignment flashcards match each diagram to the atom or ion it represents drag each item to the appropriate bin
The diagram below is called a reaction coordinate diagram. It shows how the energy of the system changes during a chemical reaction. In this example, B is at a lower total energy than A. ... The transition state is the point of highest energy between the reactant(s) and product(s). ... such as the platinum metal added to the H 2 /O 2 mixture ...
Match the diagram below to the transition metal it represents. Transition metals or inner transition metals. For the bulk metal up to 12 electrons per atom can occupy the composite band since the composite band is built up from five 3d orbitals and one 4s orbital. From wikibooks open books for an open world.
6. Which of the following compounds best fits the PMR spectrum shown below. The table shows the chemical shift for groups attached to a bromine atom or a cyano group measured to the center of the multiplet. 7. Which of the following alkyl groups would show the multiplet pattern depicted in the diagram?









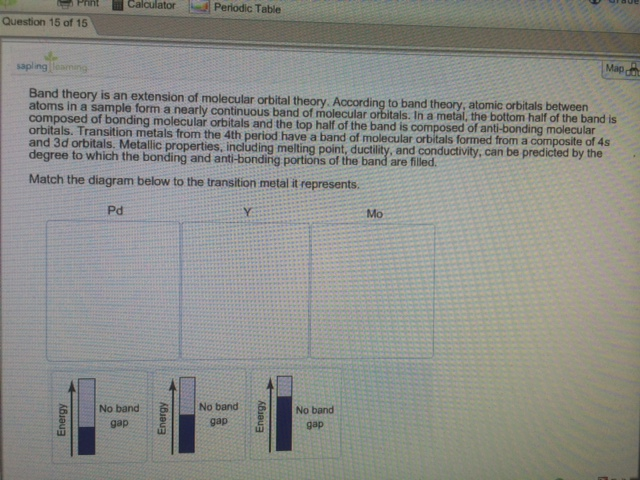












0 Response to "40 match the diagram below to the transition metal it represents."
Post a Comment