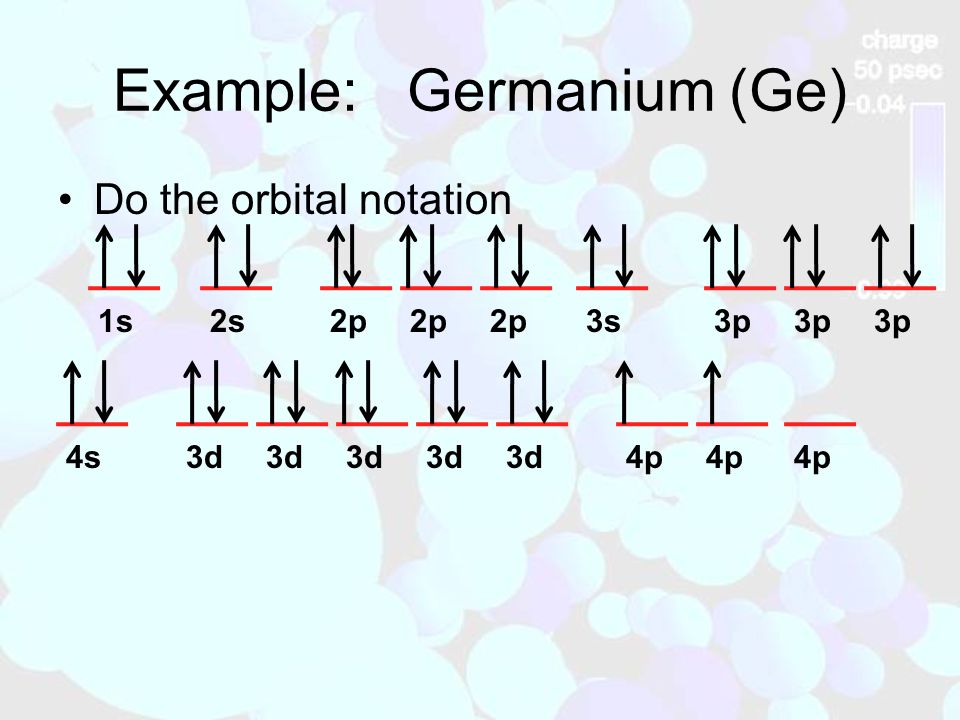
45 orbital diagram for ge
Answer (1 of 2): Are you wondering why the configuration isn't ……. [Ar] 3d10 4s2 ? Look at it from the point of view of the "last electron". Where is it? In ... Write the electron configuration and draw an orbital diagram, showing the 3s2 3p6 4s2 3d10 4p6 5s2 4d1. 7. 1s22s22p6 3s2 3p6 4s2 3dp1. Gallium. 1s. The electron configuration for Gallium, Ga is by removing electrons first from the outermost p orbitals, followed by the s orbital and finally the d. The electron configuration for Gallium, Ga is by ...
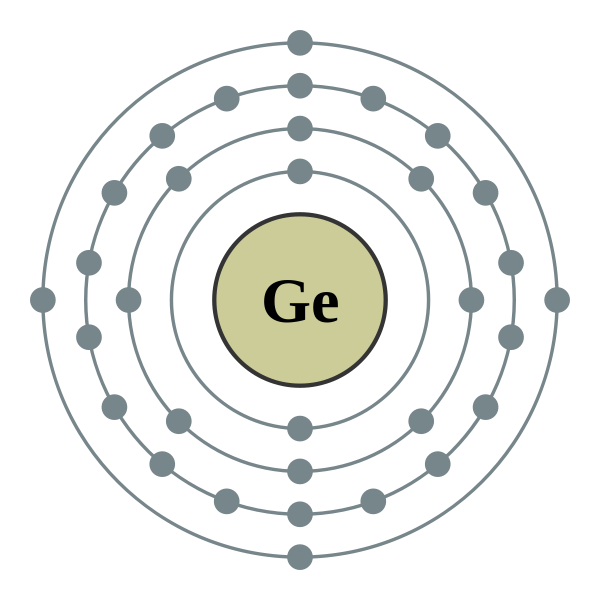
Germanium is in the same family with the elements carbon and silicon. They all have four electrons in their outer shell. The orbital structure for germanium is ...
Orbital diagram for ge
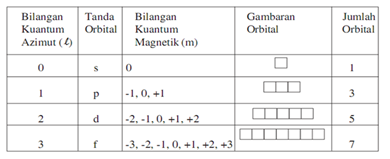
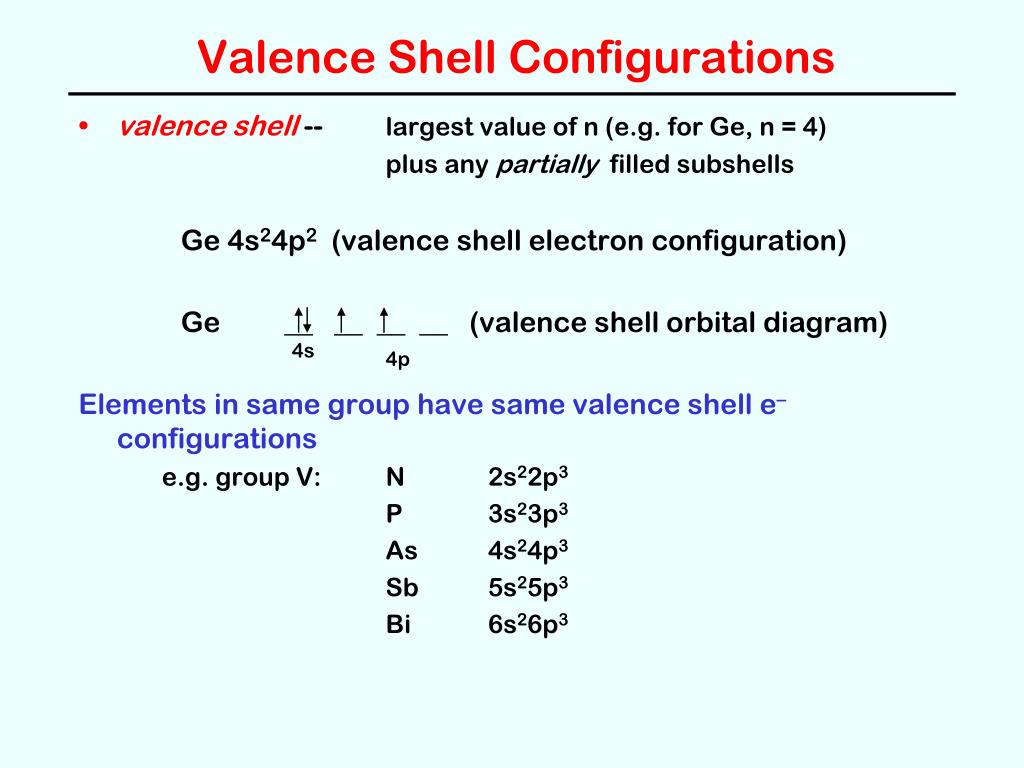
Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, "Ge": 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation. Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Then we have to think okay with the sublevels, I mean ... The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.
Orbital diagram for ge. Germanium atoms have 32 electrons and the shell structure is 2.8.18.4. The ground state electron configuration of ground state gaseous neutral germanium is [Ar] ... The orbital filling diagram for helium. The electron configuration for helium is 1s². This means that we have two electrons in the 1s orbital, which looks like this: This diagram is exactly the same as the one for hydrogen, except that there's a second arrow added to the 1s orbital. This represents the second electron in the 1s orbital, and ... The ground-state electron configurations of the elements are listed in Table 9.9.9 B. 1. The "exceptions" to the simple mnemonic noted in general chemistry texts are partly a consequence of the inadequacy of a "one-orbital order-fits-all" model. For example, copper has an electron configuration of [Ar]4s 1 d 10. germanium (Ge) in its ground state is A) A B) B C) C D) D E) E. C) C. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. C) selenium. Which ground-state atom has an electron configuration described by the following orbital
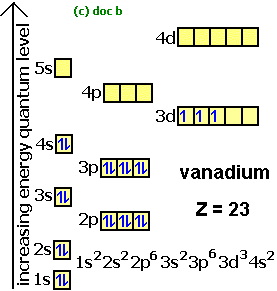
Write the orbital diagram for sulfur and determine its number of unpaired electrons. Electron configuration: 1s2 2s2 2p6 3s2 3p4. Orbital diagram: 1s= 1 up 1 down. 2s= 1 up 1 down. 2p= 1 up 1 down 1 up 1 down 1 up 1 down. 3s= 1 up 1 down. 3p= 1 up 1 down 1 up 1 up. Two unpaired electrons. Write the electron configuration for Ge. Identify the element which has the following partial orbital diagram a. Ti b. Sn c. Ge d. Zr e. Pr What is the lowest numbered principal energy level in which d orbitals are found? a. 1 b. 2 c. 3 d. 4 e. 5 All of the following electronic configurations are correct except a) 20Ca [Ar]4s2 b) 25Mn [Ar] 3d54s2 c) 29Cu [Ar] 3d104s1 d) 50Sn [Kr ... - When comparing the two elements Ga and Ge , the element with the higher first ionization energy is Ge - When comparing the two elements P and Sb , the element with the higher first ionization energy is P ... Enter the orbital diagram for the ion Zr2+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed ... Apr 28, 2021 — How many valence electrons are in GE +? ... What is germanium's atomic number? ... Electron Configuration, [Ar] 3d10 4s2 4p2.
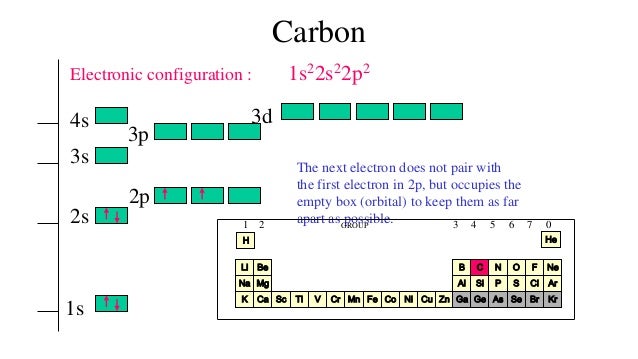
Homeline Load Center Wiring Diagram - homeline 70-amp load center wiring diagram, homeline load center 100 amp wiring diagram, homeline load center hom2-4l70 wiring diagram, Every electric arran ge ment consists of various distinct pieces. Each component ought to be set and connected with different parts in specific way. Below is the electronic diagram of the Germanium atom. Orbital diagram of the Germanium atom. Distribution of electrons over energy levels in the Ge atom The atomic number of carbon is 6, which is also the number of positively charged protons its atomic nuclei. If the atom is neutral, it will have the same number of negatively charged electrons. Its electron configuration is "1s"^2"2s"^2"2p"^2". The orbital diagram shows how the electrons are arranged within each sublevel. The maximum number of electrons allowed in an orbital is 2, each with ... MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
34. A possible set of quantum numbers for the last electron added to complete an atom of germanium (Ge) in its ground state is. Ans: C. Category: Medium Section: 7.8. 35. Electrons in an orbital with l = 3 are in a/an. A) d orbital. B) f orbital. C) g orbital. D) p orbital. E) s orbital. Ans: B Category: Easy Section: 7.6. 36.
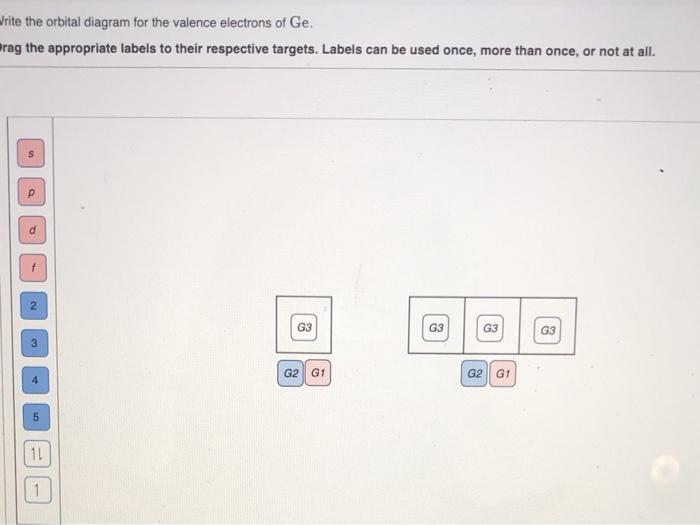
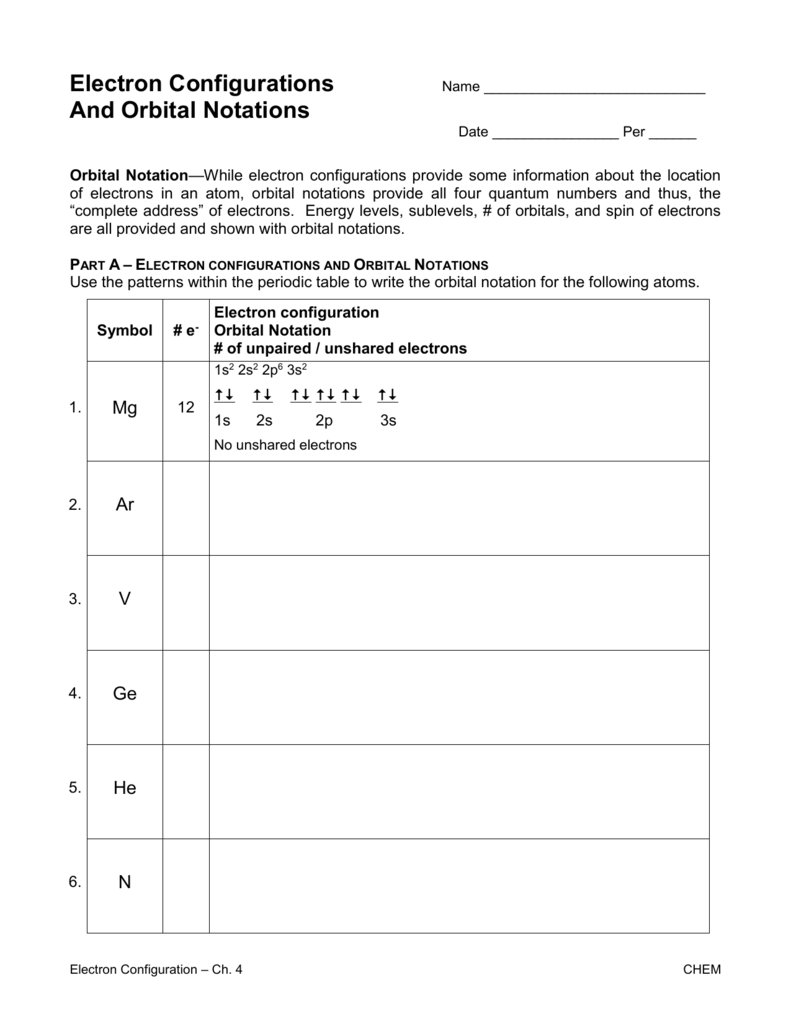
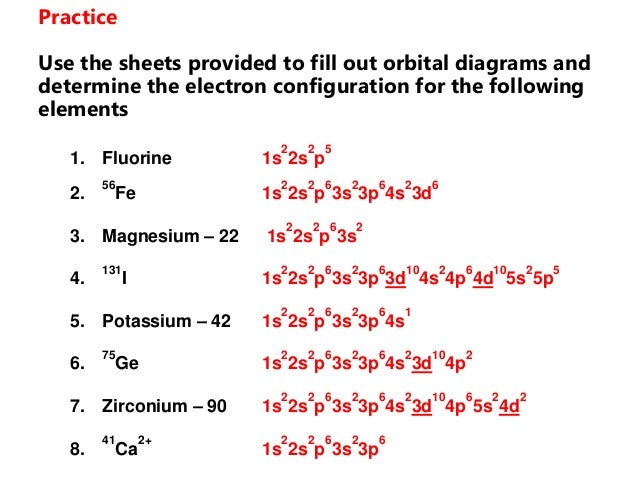
Part A - Orbital Diagrams . Use the patterns within the periodic table to draw orbital diagrams. Symbol Orbital Diagram (Don't do Nobel Gas Configuration) Mg P Ge Li Reminder of electron configuration rules: Aufbau Principle: Electrons occupy lowest energy levels first. Pauli Exculsion principle: Each orbital contains up to 2 electrons.
Chemistry Q&A Library What is the orbital diagram for the atom Geranium Ge 32. What is the orbital diagram for the atom Geranium Ge 32. close. Start your trial now! First week only $4.99! arrow_forward. Question. What is the orbital diagram for the atom Geranium Ge 32. check_circle Expert Answer.
0 Comments. on Orbital Diagram For Germanium. orbital. Because an electron can have either one of two spins, any orbital can hold a maximum of four . The orbital diagram for germanium is. 1s. 2s. 2p. 3s. Oxidation States, +4,2. Electrons Per Shell, 2 8 18 4. Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2.
Jan 28, 2021 — Germanium Electron Configuration: Ge (Germanium) is a chemical element that has a chemical symbol Ge. The atomic number of Germanium is 32.
The Electron Configuration Video Lessons. Concept: Concept: Example: Problem: Identify the element which has the following partial orbital diagram a. Ti b. Sn c. Ge d. Zr e. Pr.
Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics.
Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39: Orbital diagram of Yttrium (Y) 40: Orbital diagram of Zirconium (Zr) 41: Orbital diagram ...
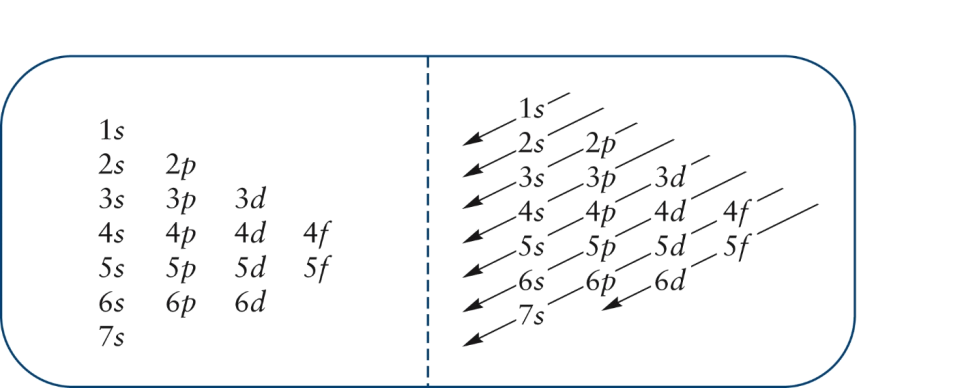
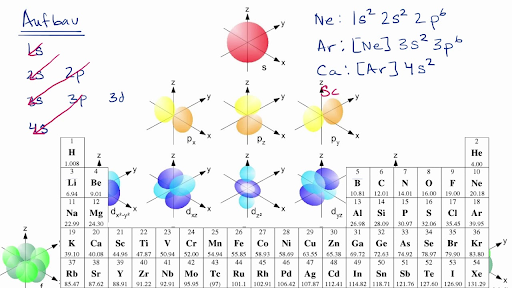
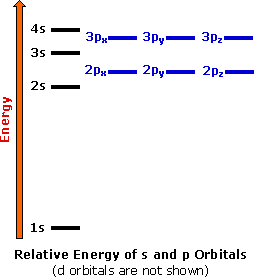
1. The Pauli principle: No more than two electrons can occupy a given orbital. If there are two electrons in an orbital, their spins must be paired (one must have m s = 1 2 and the other, m s = − 1 2). 2. The aufbau (building-up) principle: When electrons are filled in to orbitals in an atom, the orbitals with lower energy are filled first.
This chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration. It explains how to write the orbital diagram n...
1. (30 pts) For germanium (Ge) atom: 1) Write the shorthand ground state electron configuration and orbital diagram for Ge. Indicate the magnetic property (diamagnetic or paramagnetic) of the atom. 2) Write a set of quantum numbers (n. l, m, m.) for one of the germanium valence electrons. 3) Based on the above orbital diagram, predict one ...
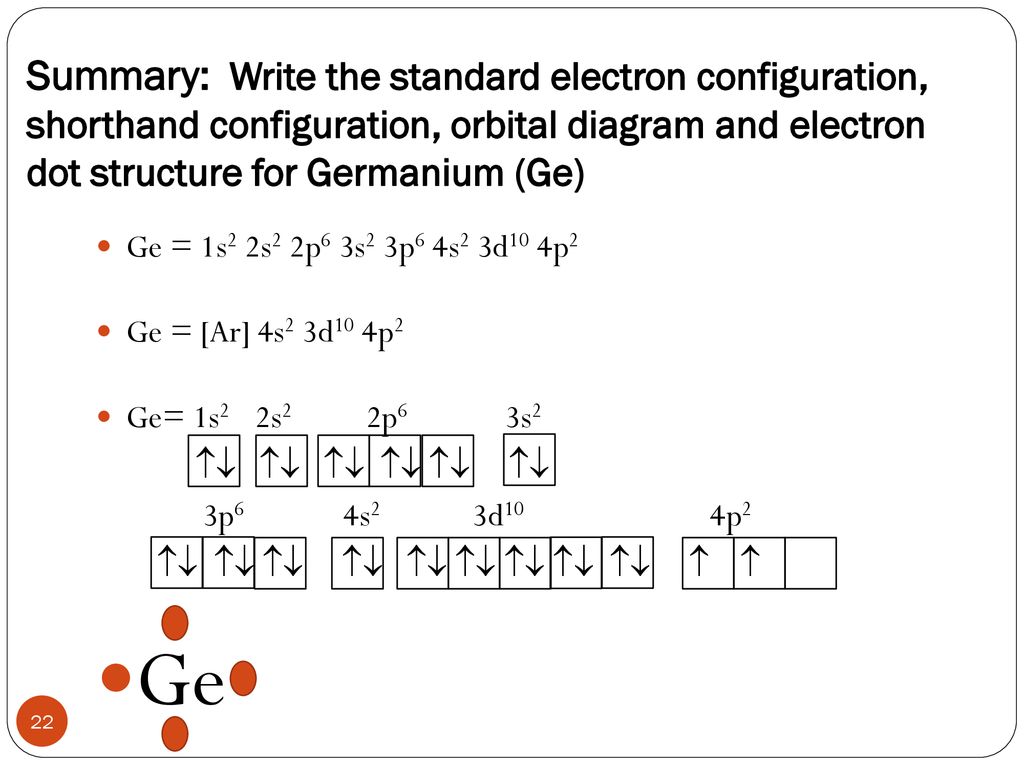
Electron Notation is: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2 Noble Gas notation is: [Ar] 4s2 3d10 4p2 Orbital Notation would be with the up & down arrow in boxes for each orbital.
A step-by-step description of how to write the electron configuration for Germanium (Ge).In order to write the Ge electron configuration we first need to kno...
orbital diagrams •Here is the orbital diagram for Lithium (Z = 3) The shorthand convention for representing the electron configuration for Lithium is •which reads "one-ess-two, two-ess-one" 22 1s22s1 Lecture 8 - Quantum Mechanical Model of the Periodic Table The orbital diagrams can also be represented horizontally: •Build up of ...
Germanium (Ge) has an atomic mass of 32. ... Electron Configuration, [Ar] 3d10 4s2 4p2. 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2. Orbital Diagram.Atomic Number: 32Group: 14 (carbon family) IVB (IUPAC), IVA ...Atomic Weight: 72.64 Isotopes
Electronic Configuration of Ge (Germanium) Atomic number of germanium is 32. Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p2 or it can be written as [Ar] 3d10 4s2 4p2. ... Orbital Diagram 1s ↿⇂ 2s ↿⇂ 2p ↿⇂ ↿⇂ ↿⇂ 3s ↿⇂ 3p ↿⇂ ↿⇂ ↿⇂ 3d ↿⇂ ↿⇂ ↿⇂ ↿⇂ ↿⇂ 4s ↿⇂ 4p ...
The fourth orbital in this subshell lies along the X and Y axes and is called the 3d x 2-y 2 orbital. Most of the space occupied by the fifth orbital lies along the Z axis and this orbital is called the 3d z 2 orbital. The number of orbitals in a shell is the square of the principal quantum number: 1 2 = 1, 2 2 = 4, 3 2 = 9.
Orbital diagrams are a pictorial description of electrons in an atom. In order to figure out where electrons go in an atom we have to follow 3 main rules. The first one being the Auf Bau Principle, the Auf Bau Principle states that each electron occupies the lowest energy orbital available. Then we have to think okay with the sublevels, I mean ...
Germanium (Ge) is located in the fourth row, group 14 of the periodic table, and has an atomic number of 32. This implies that the neutral Ge atom's electron configuration must account for 32 electrons. So, "Ge": 1s^(2)2s^(2)2p^(6)3s^(2)3p^(6)4s^(2)3d^(10)4p^(2) An alternative way of writing the electron configuration for Ge is by using the noble gas shorthand notation.























0 Response to "45 orbital diagram for ge"
Post a Comment