45 orbital diagram for neon
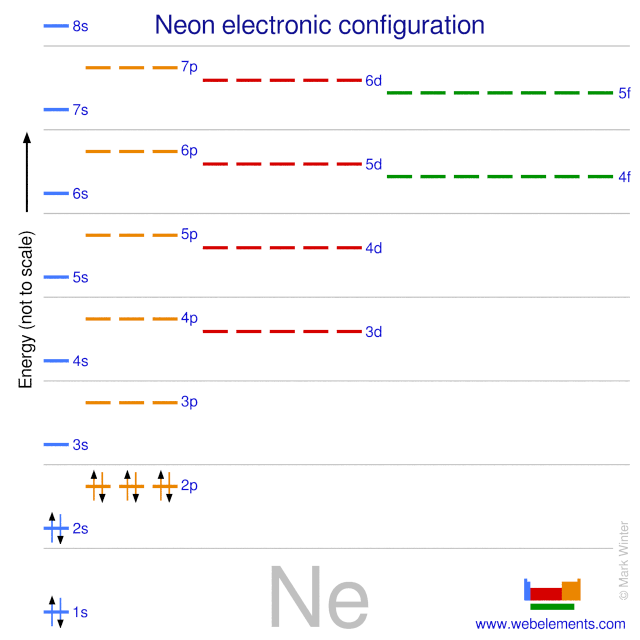
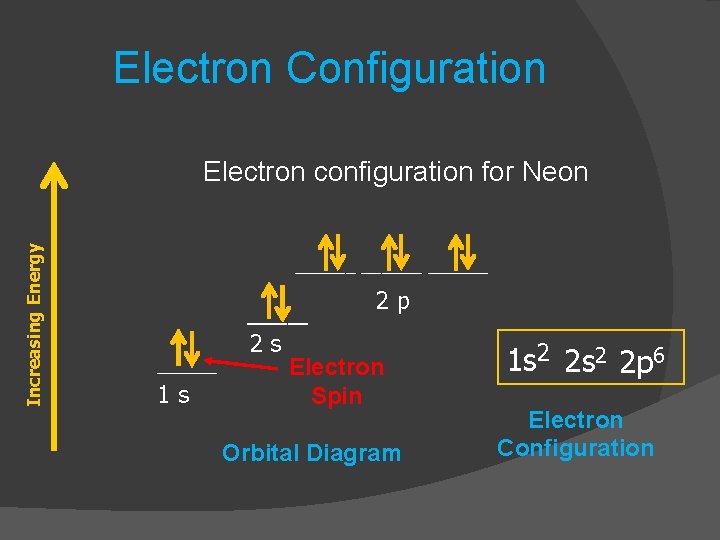
Answer and Explanation: 1. For neon, the atomic number is 10. The electronic configuration for neon is 1s22s22p6 1 s 2 2 s 2 2 p 6 . The full orbital diagram for neon is shown below. Orbital diagram. Neon electron configuration is 1s 2 2s 2 2p 6.The symbol for neon is ‘Ne’ and it is an inert element. This article gives an idea about the electron configuration of neon(Ne) and orbital diagram, period and groups, valency and valence electrons of neon, application of different principles. The tenth element in the periodic table is neon.
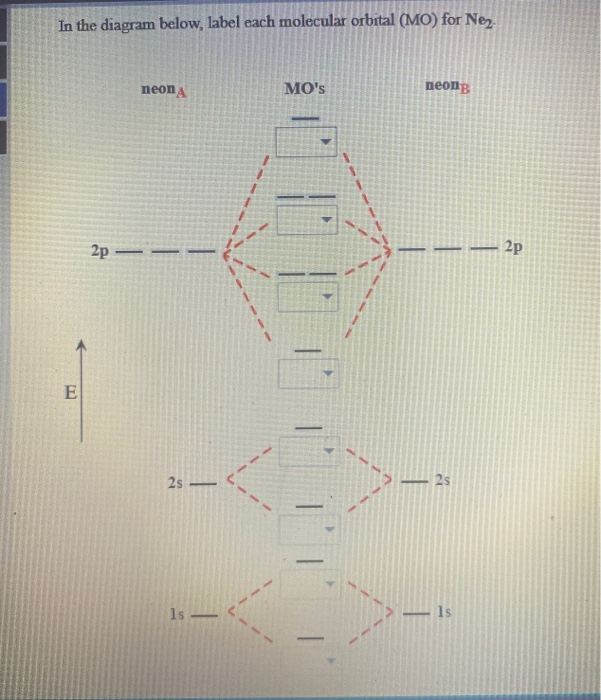
Nov 17, 2021 · Molecular orbital diagram practice worksheet
Orbital diagram for neon
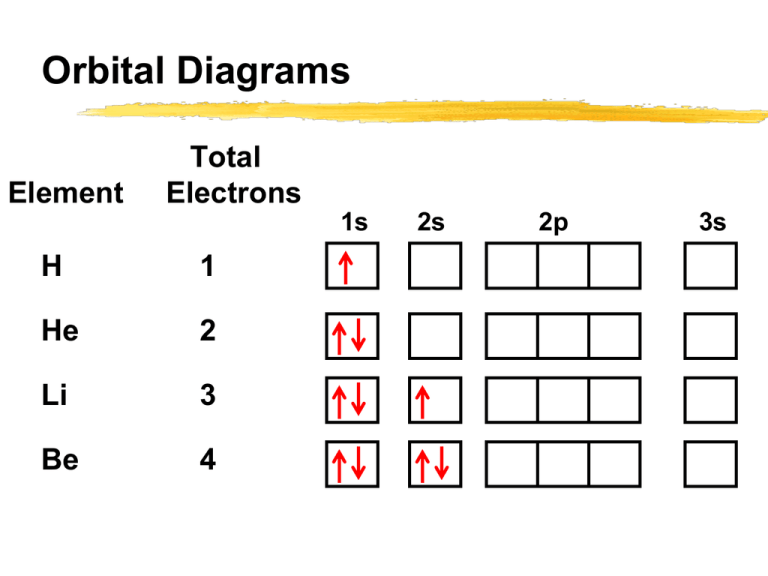
Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. Therefore the Ne electron configuration will be 1s ... The elements from boron to neon in the periodic table are filling the 2p orbital. The three possibilities for 2p orbitals can be associated with spatial directions, say x,y and z. The order of filling will place one electron in each before placing two in any one orbital since the … Sep 16, 2021 · Thus, a p-orbital has three possible orientations (-1, 0, 1 magnetic quantum numbers). Neon, the last element in the main second energy level, has 10 electrons. How are these electrons distributed and located? The first 2 electrons go in the 1s-orbital, or …
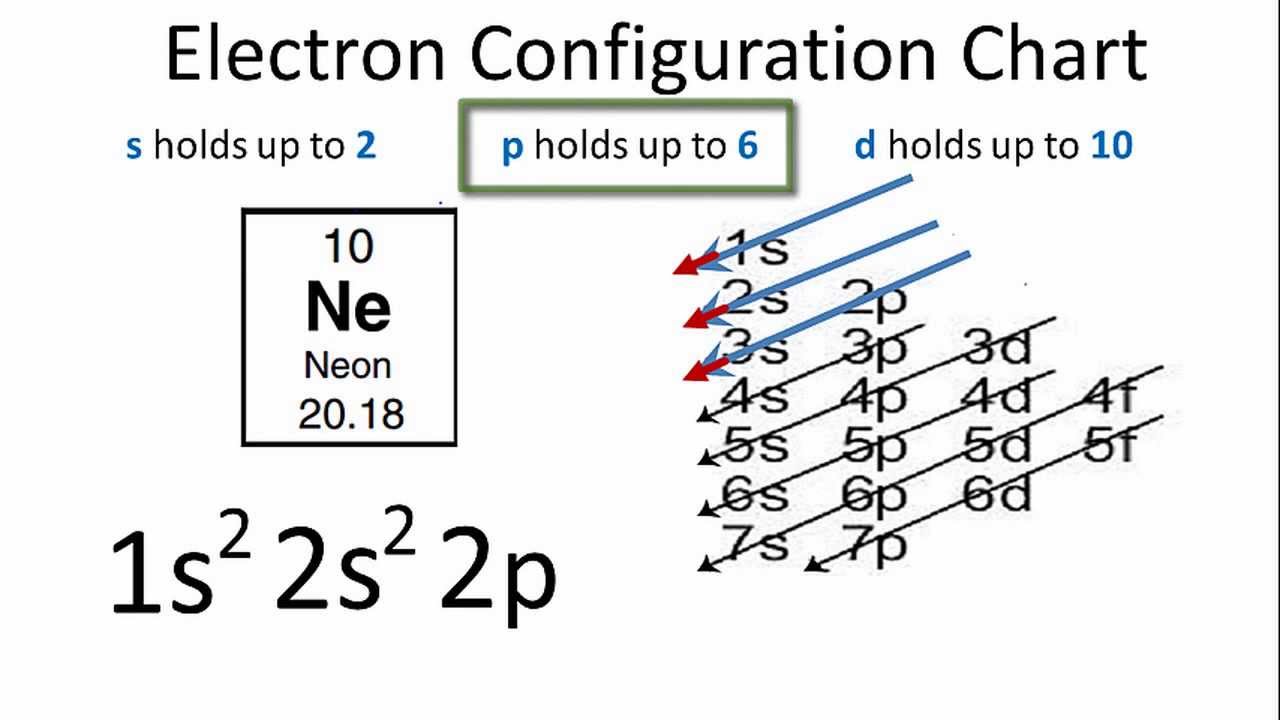
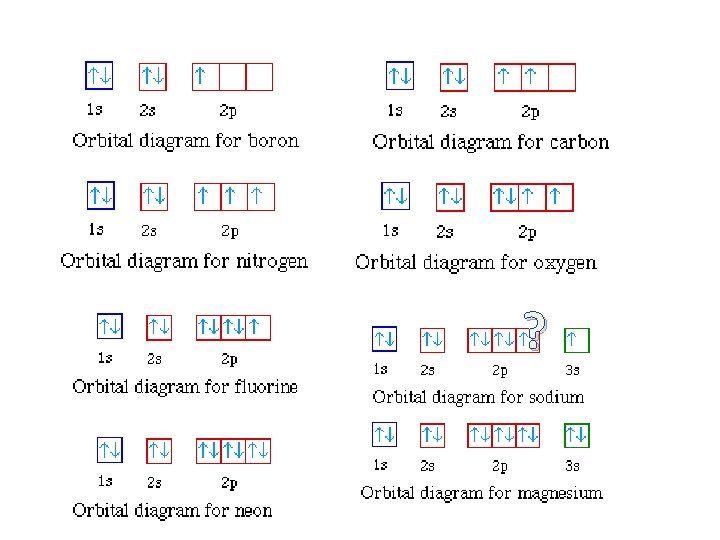
Orbital diagram for neon. Boron (atomic number 5) has five electrons. Four electrons fill both the 1s and 2s orbitals. The fifth electron is added to a 2p orbital, the sublevel next higher in energy (Figure 5.9). The electron configuration of boron is: B: 1s 2 2s 2 2p 1. Table 5.2 shows the electron configurations of the elements with atomic numbers 1 through 18. Fluorine electron configuration is 1s 2 2s 2 2p 5.The symbol for fluorine is F. The period of fluorine is 2 and it is a p-block element. The electron configuration of fluorine(F) and the orbital diagram is the main topic of this article. Oxygen electron configuration is 1s 2 2s 2 2p 4.The period of oxygen is 2 and it is a p-block element. This article gives an idea about the electron configuration of oxygen(O) and orbital diagram, period and groups, valency and valence electrons of oxygen, bond formation, compound formation, application of different principles. The eighth element in the periodic table is oxygen. [2,8] or 1s^2 2s^2 2p^6 Neon has atomic number 10, so a neon atom has 10 protons in its nucleus and therefore 10 electrons. The n=1 shell can only hold 2 electrons, so the remaining 8 electrons fill the n=2 shell. The more detailed version of the electronic configuration reflects the sub-shells in the n=1 and n=2 shells, where the n=1 shell just has a single s-sub shell containing a single s ...
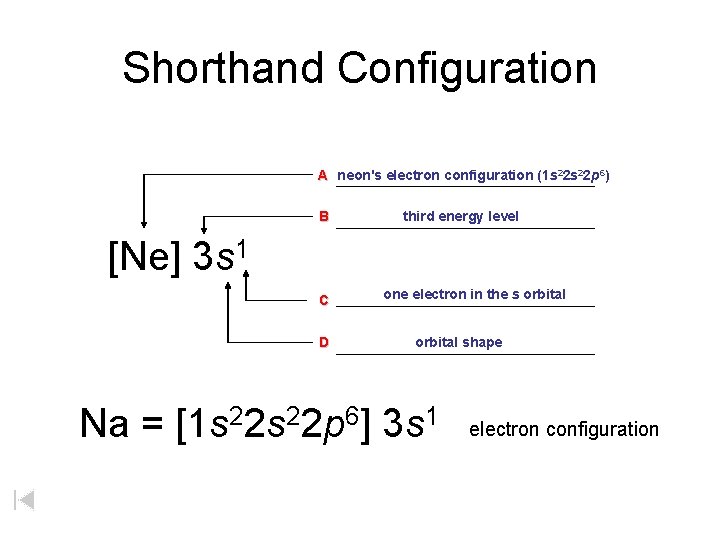
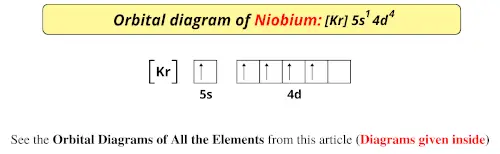
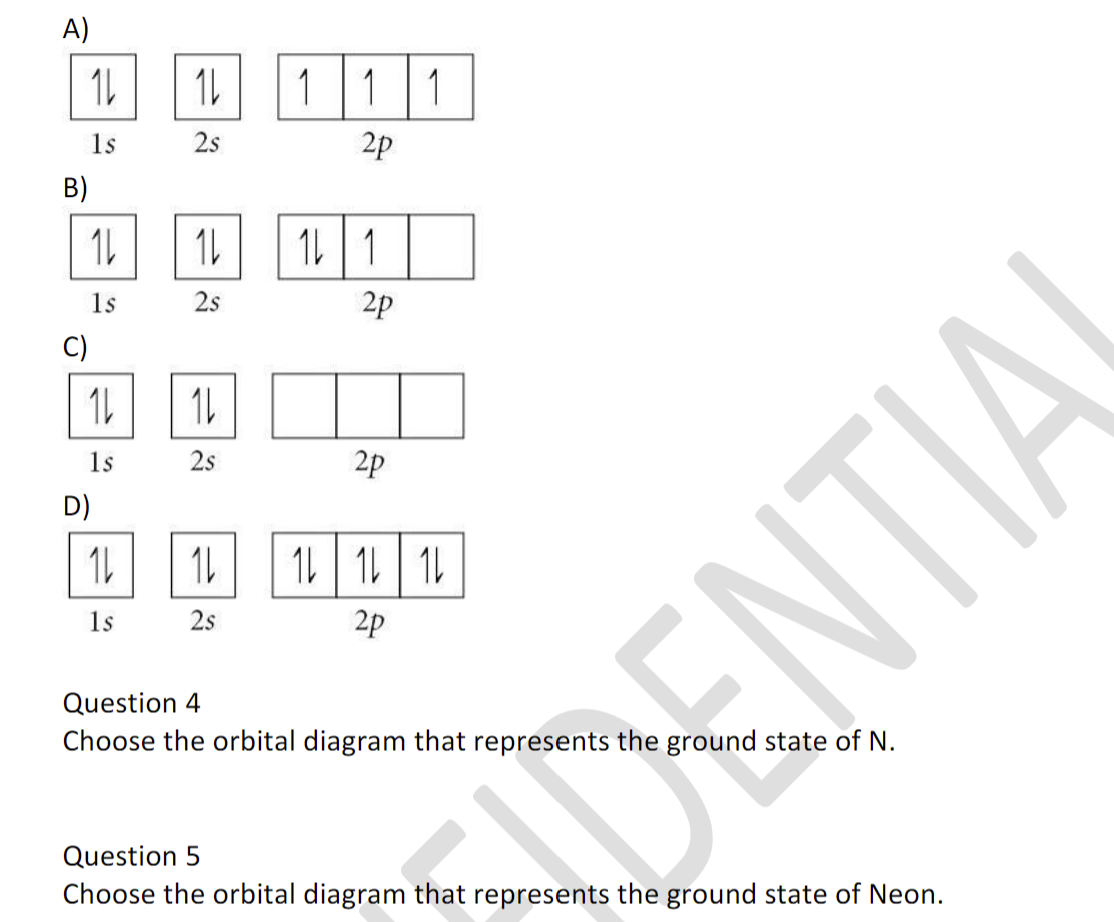
The orbital diagrams for fluorine and neon are shown. The next two electrons continue to pair those electrons that are unpaired to fill up the 2p orbitals. With neon the second level is filled with electrons. Completed levels are a characteristic of all noble gases. If we look at the energy level diagram for neon the completed second level ... Write the full electron configuration, noble gas electron configuration, and fill in the orbital diagrams, for the following elements. 1. Nitro en 3s B 2. Chlorine Is 3. Sodium a øan a as ap 4. Neon Is 23 5. Nickel 6 Vanadium Is 7 Cop er The electron configuration of neon is: 1s22s22p5. An orbital diagram is similar to electron configuration, except that instead of indicating the atoms by total numbers, each orbital is shown with ... An orbital diagram, or orbital box diagram, is a way of representing the electron configuration of an atom. A box, line, or circle, is drawn to represent each orbital in the electron configuration (using the ... Neon : atomic number (Z) = 10 (p block element)
In atomic theory and quantum mechanics, an atomic orbital is a mathematical function describing the location and wave-like behavior of an electron in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus.The term atomic orbital may also refer to the physical region or space where the electron can be ... Fill in the orbital energy diagram for the neon atom. 2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining ; Question: Fill in the orbital energy diagram for the neon atom. 2p El 2s 1s Submit Answer Try Another Version 2 item attempts remaining . This problem has been solved! Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11 ... The correct orbital diagram, obeying Hund’s Rule, will note the two 2p electrons to be unpaired in two of the three available orbitals, both with “spin-up.” Since electrons always occupy an empty orbital before they fill up, it would be incorrect to draw the two 2p electrons in …
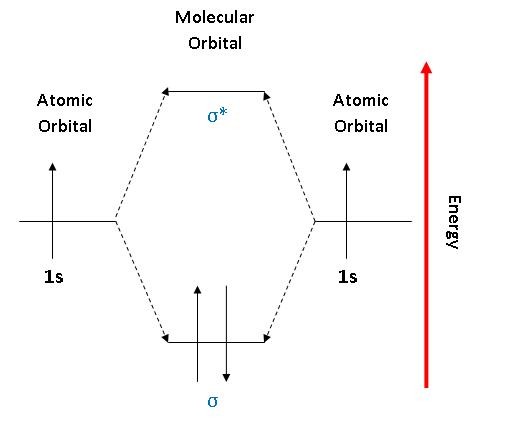
Molecular Orbital Diagram of Neon Molecule Video Lecture from Chapter Nature of Chemical Bond of Subject Chemistry Class 11 for HSC, IIT JEE, CBSE & NEET.Wat...
Sep 16, 2021 · Thus, a p-orbital has three possible orientations (-1, 0, 1 magnetic quantum numbers). Neon, the last element in the main second energy level, has 10 electrons. How are these electrons distributed and located? The first 2 electrons go in the 1s-orbital, or …
The elements from boron to neon in the periodic table are filling the 2p orbital. The three possibilities for 2p orbitals can be associated with spatial directions, say x,y and z. The order of filling will place one electron in each before placing two in any one orbital since the …
Neon is the tenth element with a total of 10 electrons. In writing the electron configuration for neon the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Ne go in the 2s orbital. The remaining six electrons will go in the 2p orbital. Therefore the Ne electron configuration will be 1s ...
























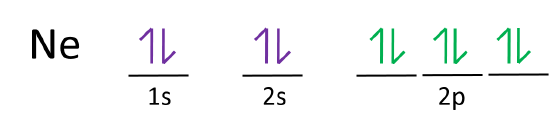



0 Response to "45 orbital diagram for neon"
Post a Comment