42 molecular orbital diagram for ne2 2+
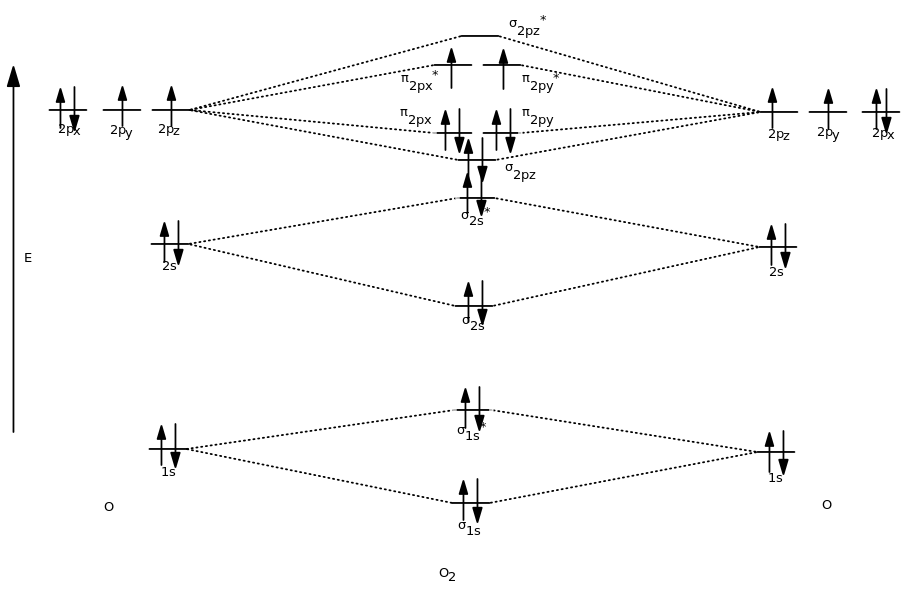
Molecular orbital diagram of H 2 (Hydrogen molecule). Molecular Orbital Diagrams of Diatomic Molecules Introduction: In chemistry molecular orbital (MO) theory is a method for determining molecular structure in which electrons are not assigned to individual bonds between atoms, but are treated as moving under the influence of the nuclei in the ... Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
FREE Expert Solution. We are asked to determine the bond order from the molecular orbital diagram of Ne2 and to check whether the calculated bond order agrees with the Lewis structure of Ne2. Part A. Draw the Lewis Structure of Ne2. Part B. Determine the bond order from the molecular orbital diagram of Ne2. Part C. Explain whether the bond ...

Molecular orbital diagram for ne2 2+
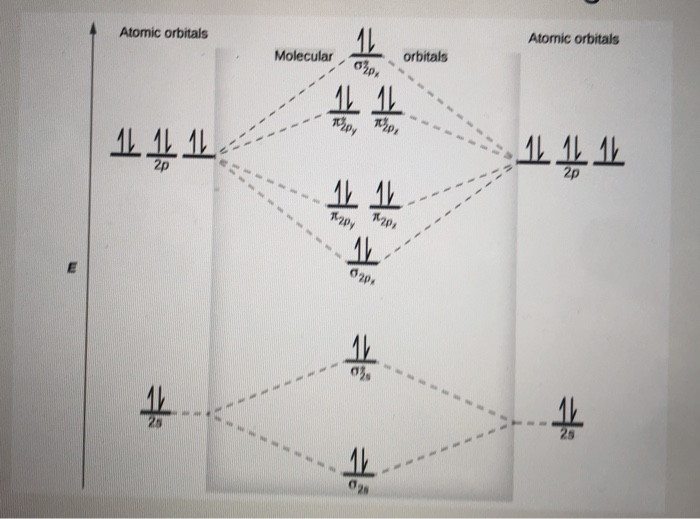
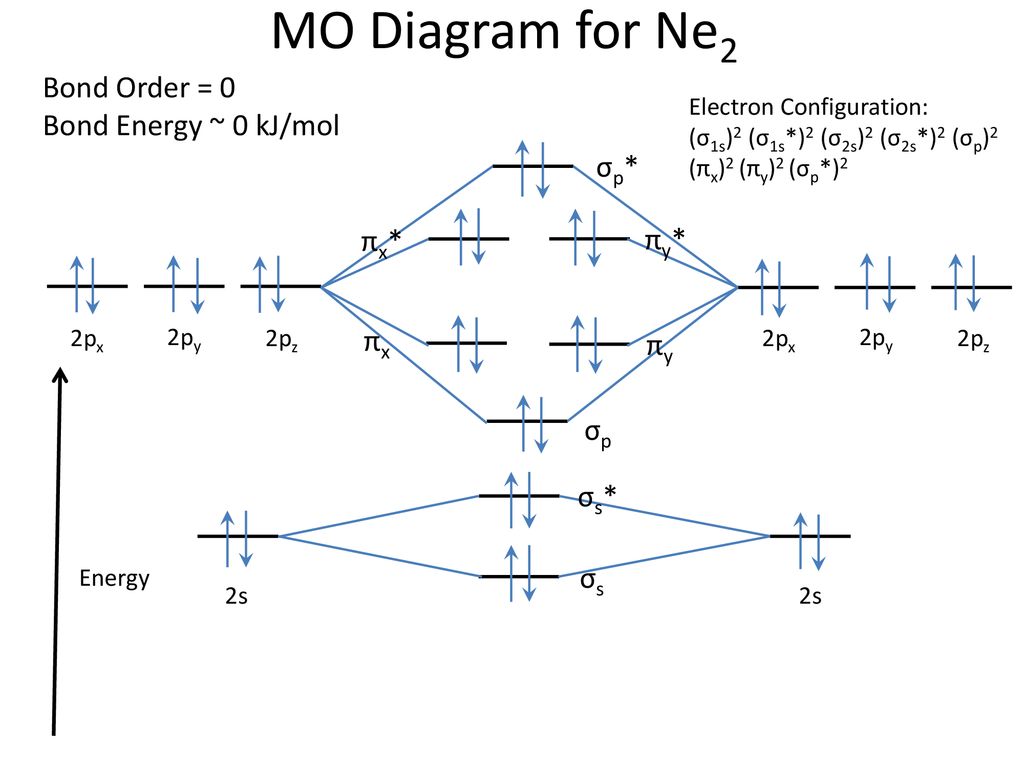
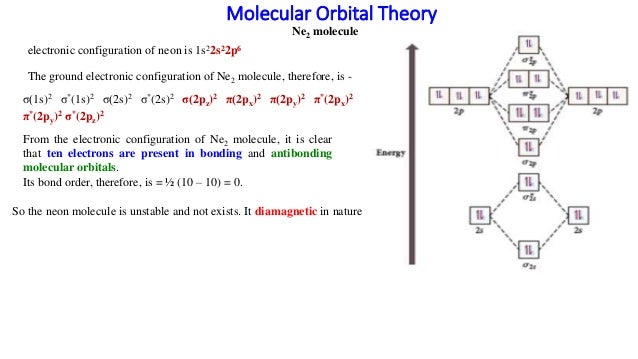
Hence the bond order of Ne2 according to M.O.T is. =(Nb-Na)/2. =(10-10)/2. (since,both bonding orbitals and non-bonding orbital contains 10 electrons).4 answers · 5 votes: Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π ... Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
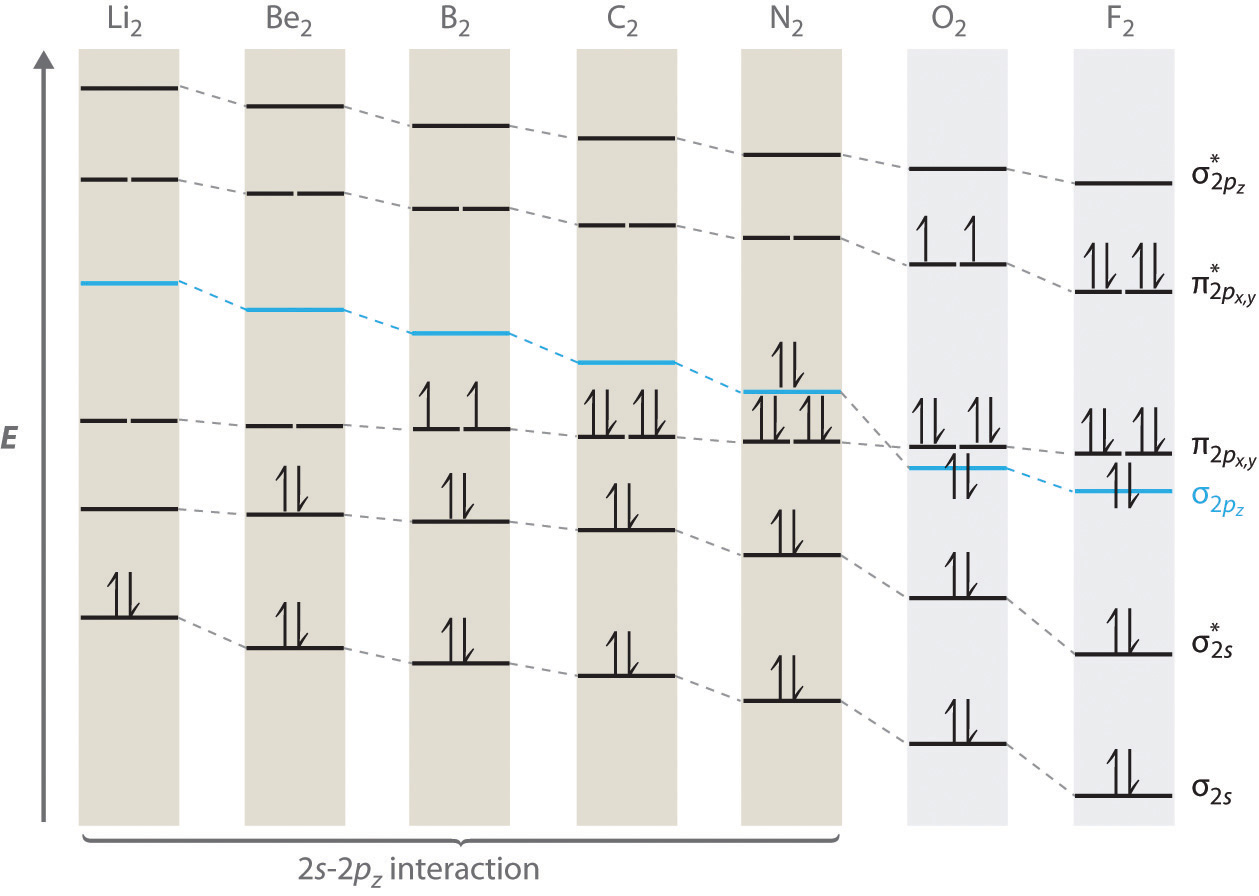
Molecular orbital diagram for ne2 2+. H2−2 Ne2 He2+2 F2−2. will not exist will not exist will exist ... in the diagram showing orbital overlap below. Draw an appropriate Lewis structure for CH2CHCH3. ... Based on the molecular diagram, classify each change as physical or chemical. Physical Change - a - c Chemical Change - b. Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ... 15 F2 Molecular Orbital Diagram. We assume that the electrons would fill the molecular orbitals of molecules like electrons fill atomic we will use this diagram to describe o2, f2, ne2, co, and no. The lowest energy unoccupied molecular orbital is 2p_ (sigma), so that is where the extra electron will be added. Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ...
Draw the molecular orbital diagram for Ne2+ and determine if the bond ... In Ne2 ion, the electronic configuration of Ne is Ne 1s2s22p6 Calculate the total ... The molecular orbital electronic configuration, Magnetic property: Since bond order is zero, Be 2 molecule does not exist. It is diamagnetic due to the absence of any unpaired electron. B 2 molecule: The electronic configuration of B atom (Z = 5) is. B 2 molecule is formed by the overlap of atomic orbitals of both boron atoms. A number of ... Molecular-orbital energy patterns for homonuclear diatomic molecules. ... and 02 (B) F2 and Ne2 (C) 02 and B2 (D) C2 and N2 Q. 10 5 Which of the following is correct ? ... HF (d) HCl, based upon ... 21/11/2018 · Mar 4, Find an answer to your question Draw and explain the molecular orbital diagram of Ne2. On the basis of molecular orbital diagram, explain. According to Molecular Orbital theory, only those molecule can exists which have net positive bond order while the molecules with negative or.
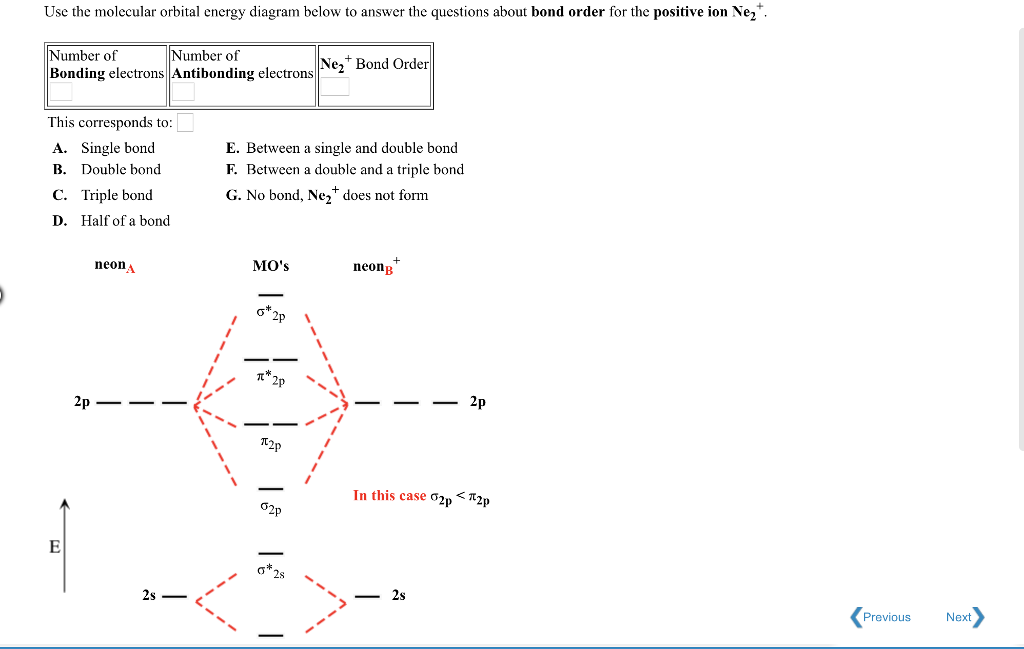
The Lewis structures have an unpaired electron and an average bond order of 1.5. O2 has two unpaired electrons in its π* orbitals, and a bond order of 2. The ...29 pages The molecular orbital diagram of hypothetical molecule is given in the attachment. Using the MO diagrams shown in Figure 8. Be and Ne molecules would have a bond order of , and these molecules do not exist. H (Total electrons = 2), Therefore B. The addition of two more electrons to make Nefills all the bonding and antibonding MOs. the pi(2p) bonding orbitals are LOWER than the sigma(2p) bonding orbitals.N2(2-) has a bonding order of 2, which predicts that there will be a stable double ... what is the bond order of Ne2 2+. i know its 1 but I do not know how to use this diagram. can someone explain this? Show transcribed image text. Expert Answer.
Answer (1 of 4): Neon is denoted by Ne which is noble gas. Noble gases are non-reactive by nature.The outermost shell in Ne element is fully filled with electrons where the electron is 10 in number. This means that will have 20 electrons. This is why it does not need to react with any other atom ...
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
Mar 4, 2018 — Explanation: Neon atom has 10 electrons and its electronic configuration is . When molecule is considered, it has two neon atoms and thus is ...2 answers · 18 votes: Bond order of Ne2 = (10-10) =0 So Ne2 is unstable ;Ne2 cannot exist

Draw And Explain The Molecular Orbital Diagram Of Ne2 On The Basis Of Molecular Orbital Diagram Brainly In
A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. According to the molecular orbital theory, in a supposed He2 molecule, both the if we draw its MOT DIAGRAM, 2 e's enter the Bonding molecular Orbital and 2 .
For Ne2, construct three molecular orbital diagrams, one each for the neutral molecule, the +1 cation, and the -1 anion. Give each MO an appropriate label. Determine the electron configuration and bond order for each, and rank the three species in order of increasing bond order. Rationalize the trend in bond order in terms of bond strength.
Academia.edu is a platform for academics to share research papers.
After reading the theory part draw the MO diagrams for the ... H2, B2, C2, N2, O2, Ne2, F2 ... 2. Number of electrons in antibonding orbitals.13 pages
A) O2^2-B) Ne2^2+ C) O2^2+ D) F2^2+ E) None of the above are paramagnetic; 3) Draw the molecular orbital diagram needed, and determine which of the following is paramagnetic. A) B2^2+ B) B2^2-C) N2^2+ D) C2^2-E) B2; 4) Draw the molecular orbital diagram shown to determine which of the following is most stable.
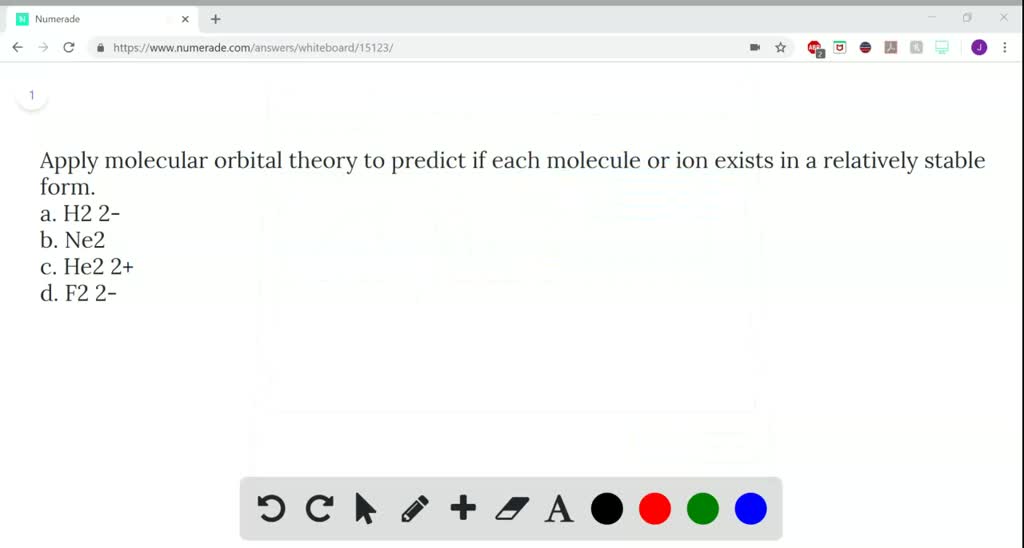
From Molecular Orbital Diagram, which is most stable? A. F2 2-B. Ne2 2+ C. O2 2+ D. F2 E. F2 2+ C. O2 2+ Choose the compound below that should have the highest melting point according to the ionic bonding model. A. CaS B. NaCl C. RbI D. MgO E. AlN. E. AlN.
There are two MO diagrams you need to memorize for diatoms (N2, O2, Ne2, etc).One is for the elements up to Nitrogen. The other is for AFTER nitrogen (start...
0:21 Molecular Orbital Diagram of Oxygen Molecule3:30 Molecular Orbital Diagram of Florine Molecule5:25 Molecular Orbital Diagram of Neon MoleculeSo as we d...
Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ...
Answer (1 of 4): The atomic number of fluorine is 9, so a (neutral) F2 molecule has a total of 18 electron, or 14 valence electrons (excluding the four 1s electrons). The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th...
In this section, we will compare MO diagrams for diatomic molecules X-X, from Li 2 to Ne2. We will predict their bond order and see how the. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining . However, experimental and computational results for homonuclear diatomics from Li2 to N2 and certain heteronuclear ...

Write The Molecular Orbital Structure For The Ne2 At Nr 10 Molecule Does This Molecule Exist Homeworklib
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
01/05/2021 · These hybrid orbitals have square planar geometry. For example [Ni(CN) 4] 2-ion. Molecular Orbital Theory: Molecular orbital theory was developed by Hund and Milliken in 1932. The basic idea of molecular orbitals theory is that atomic orbitals of individual atoms combine to form molecular orbitals.
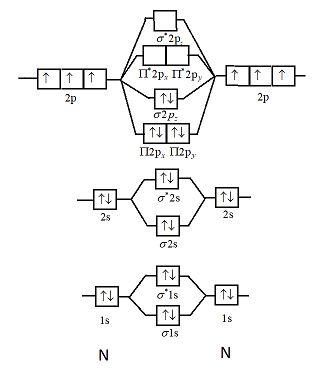
... be expected to have a triple bond, F2 , a single bond and Ne2 , no bond. ... Use the molecular orbital energy level diagram to show that N2 would be ...1 answer · Top answer: Formation of N2 molecule: Electronic Configuration, σ 1s^2<σ *1s^2<σ 2s^2<σ *2s^2<[pi 2px^2 = pi 2px^2]<<σ 2pz^2 Bond order = (Nb - Na)/2 = (10 - ...

Why Ne2 Molecule Does Not Exst Chemistry Chemical Bonding And Molecular Structure 6210904 Meritnation Com
Academia.edu is a platform for academics to share research papers.
Molecular Orbital Diagram for Nitrogen Gas (+1 ion) (N2(+)).Fill from the bottom up, with 9 valence electrons total.Bonding Order is 2.5, so it is unstable, ...
Molecular orbital diagram for ne2 2
With the help of molecular orbital diagram show that Ne2 cannot exist as stable molecule. (Atomic number of Ne = 10).
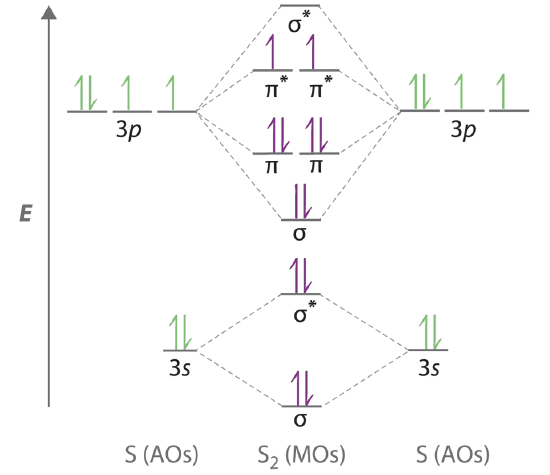
Draw the molecular orbital energy level diagram for each of the following species Be2+, Be2, and Be Indicate theirnumbers of unpaired electron and mention their magnetic diagramweb.netate their bond orders, and state which species is moststable% (1). Molecular orbitals of Li 2, Be 2, to F 2 The molecular orbital theory (MO) has been introduced ...
A) The total number of molecular orbitals formed doesn't always equal the number of atomic orbitals in the set. B) A bond order of 0 represents a stable chemical bond. C) When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular ...
Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron.
Question 35. Use molecular orbital theory to explain why the Be 2 molecule does not exist. Answer: Question 36. Compare the relative stability of the following species and indicate their magnetic properties: O 2, O 2, O 2 – (Superoxide),O 2 2-(peroxide) Answer: O 2 — Bond order = 2, paramagnetic O 2 + — Bond order = 2.5, paramagnetic

Draw The Molecular Orbital Diagram For Ne2 And Determine If The Bond Between The Two Atoms Homeworklib
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways. Page 1. MO Diagrams for Elements Li2 through Ne2. (Don't memorize.) Li2 through N2. O2 through Ne2.
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Even rather simple molecular orbital (MO) theory can be used to predict from the bottom of the diagram because this is how MO diagrams are constructed, from N2, O2, F2, Ne2 the complexity of the molecular orbitals develop in two ways.Draw the molecular orbital diagram for Ne 2 + and determine if the bond between the two atoms will be stable.
Hence the bond order of Ne2 according to M.O.T is. =(Nb-Na)/2. =(10-10)/2. (since,both bonding orbitals and non-bonding orbital contains 10 electrons).4 answers · 5 votes: Molecular orbital confuguration of Ne2 is σ1s²σ*1s²σ2s²σ*2s²σ2Pz²π2Px²π2Py²π*2Px²π ...

Use The Molecular Orbital Energy Level Diagram To Show That N2 Would Be Expected To Have A Triple Bond F2 A Single Bond And Ne2 No Bond















0 Response to "42 molecular orbital diagram for ne2 2+"
Post a Comment