40 molecular orbital diagram for cl2
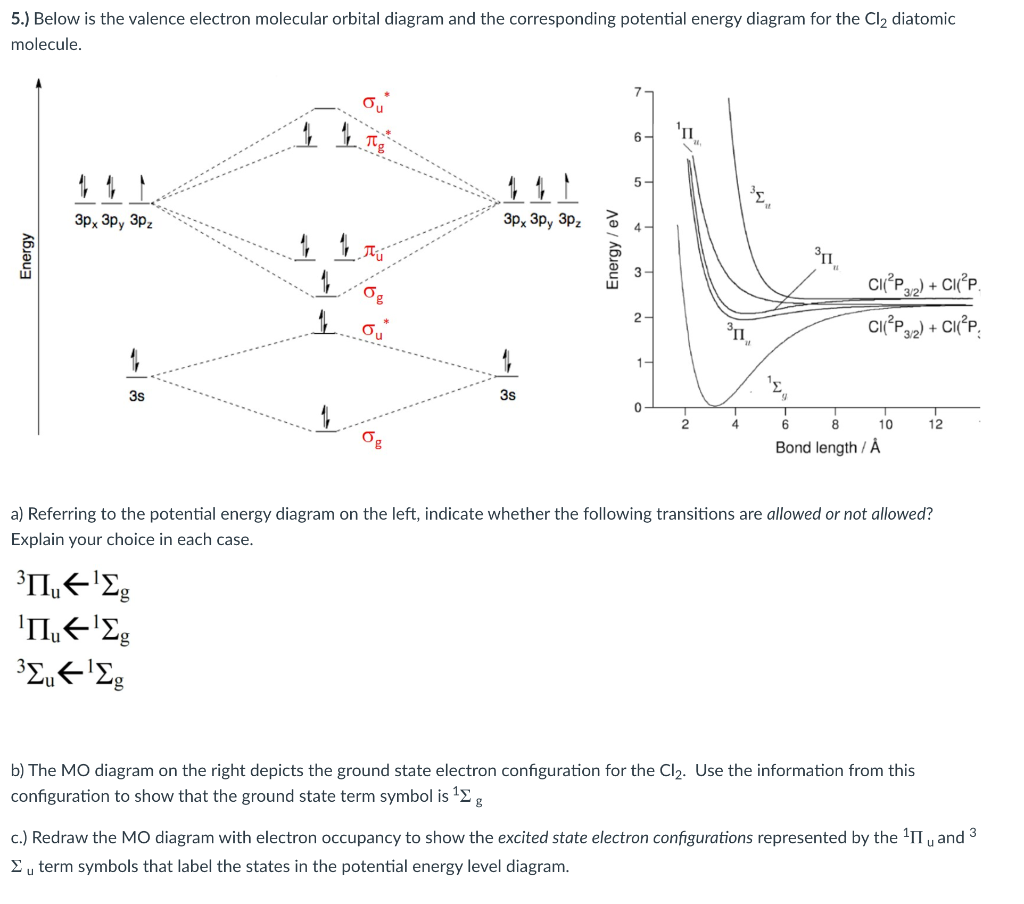
Below is the valence electron molecular orbital diagram and the corresponding potential energy diagram for the Cl2 diatomic molecule. 'n 5- Зр, Зр, Зр. 3p, 3p, 3P. CIP) + CI 2- CIP) + CI 3s 3s 10 12 Bond length / Å a) Referring to the potential energy diagram on the left, indicate whether the following transitions are allowed or not allowed? MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br ...
Molecular orbital diagram for cl2
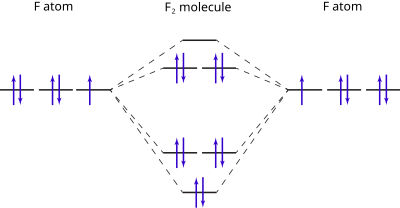
Molecular Orbital Diagrams. An MO diagram, just like an atomic orbital diagram, shows the relative energy and number of electrons in each MO. The MO diagram also shows the AOs from which each MO is formed. Bond order is calculated as follows: ½[(# of e-in bonding MO) - (# of e-in antibonding MO)] According to Molecular Orbital Theory, its electronic configuration is as follows: $1s2 $*1s2. CH4 + 2 Cl2--> CH2Cl2 + 2HCl Cl-Cl bond energy: 242 kj/mole 1. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. Identify HOMO and LUMO. Answer (1 of 2): B.O. of Cl2 = 1
Molecular orbital diagram for cl2. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Cl2 molecular orbital diagram. In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. Energy of. Cl atom has 17 electrons, so chlorine molecule has (Cl2) has 34 electrons. so, bond order of chlorine molecule is 1. Jun 5, 2016 · 1 answerThe bond order is 0.5. Explanation: We have to construct the molecular orbitals for the Cl-2 ion. The electron configuration of Cl is ... ClO2 molecular orbital diagram? Ask Question Asked 2 years, 10 months ago. Active 2 years, 9 months ago. Viewed 1k times -1 $\begingroup$ I was trying to construct a MO diagram for ClO2, and since I'm not too sure about my final result I thought I'd check it from one of the experts here. ClO2 is of C2v point group, so I just read off the C2v ...
COCl2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. COCl2 is a chemical compound, known by the name 'phosgene'. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 98.92 gram/mol. It is non-flammable in nature and bears a suffocating odor. Question: Draw the molecular orbital diagram for Cl2. Assume 3s and 3p orbitals show similar LCAO as 2s and 2p orbitals (Label increasing energy using an arrow next to the diagram. Label each atomic and each molecular orbital) Calculate the bond order and give the molecular orbital configuration for the valence electrons. The molecular orbital diagram of CN molecule is shown in the following figure: Reference: Essentials of Physical Chemistry /Arun Bahl, B.S Bahl and G.D. Tuli / multicolour edition. Molecular Orbitals for Heteronuclear Diatomic Molecules (MO Theory) Reviewed by تعرف على علم الكيمياء on 5/20/2019 Rating: 5 What is a molecular orbital energy diagram? ... F2, Cl2, Br2, and I2. Is CN paramagnetic? CN- has an extra electron. This pairs up with the electron in the highest occupied σ-orbital. As all the electrons are now paired, CN- is diamagnetic (it is weakly repelled by a magnetic field). CN is paramagnetic whereas CN- is diamagnetic.
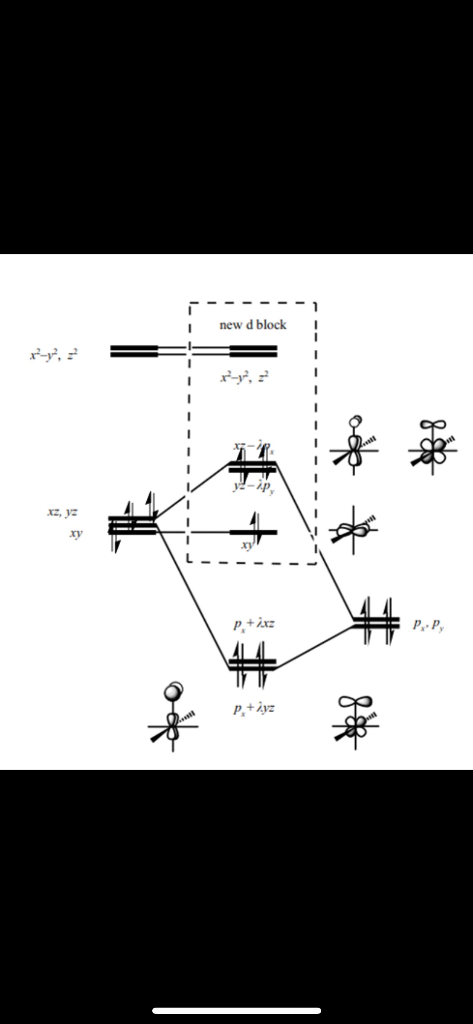
Molecular Orbital Diagram Maker. These quizzes enable you to build your own molecular orbital diagram from components. A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F. ... FREE Answer to (a) Draw a molecular orbital energy diagram for Cl2 and show which orbitals are occupied with electrons. (b) How many ba...2 answers · Top answer: 1 1 1 1L 1L 1 I .. 32 34. 3P AL * IL → 35 : 35 this electronie configuration of CL = ... This video creates the full molecular orbital diagram for dichlorine, shows the coordinate system, the point group, the symmetries of the atomic orbitals, th...
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
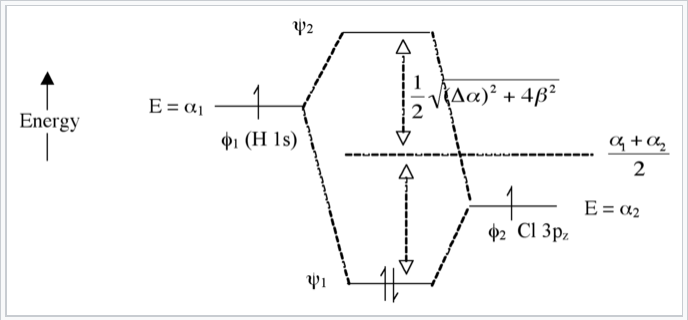
The bond order is Figure The molecular orbital energy-level diagram for both the NO+ and CN-ions. Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The. Molecular geometry of Cl2 Hybridization of Cl2.
Through the molecular geometry diagram, one can study hybridization, polarity, and molecular orbital structure of the molecule determining the behavior of the valence electrons undergoing bond formation. The bond angle between oxygen and chlorine atoms (O-Cl) is 110.9° which gives the molecule a bent or V-shaped.
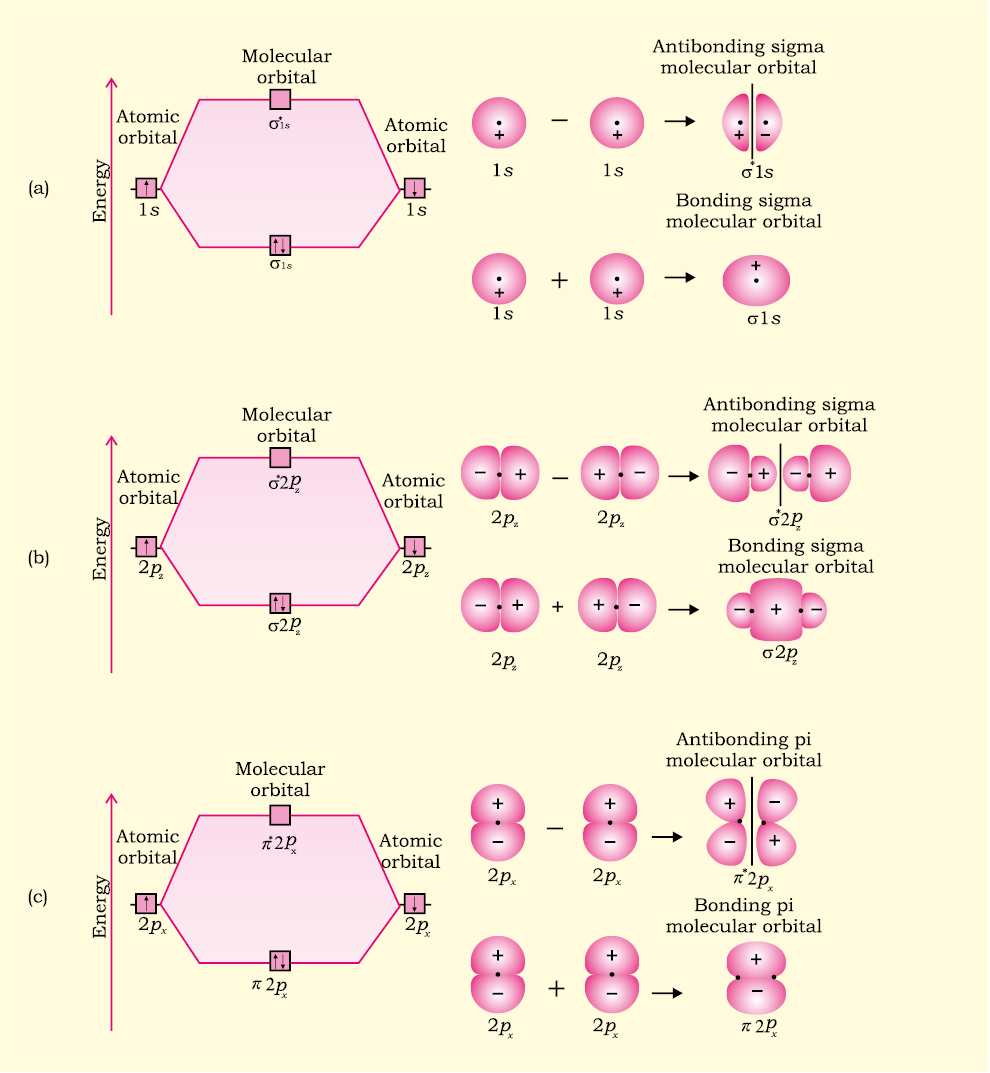
Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ...
The S orbital energies are -22.7 eV (3s) and -11.6 eV (3p); the 1s of H has an energy of -13.6 eV. Because of the difference in their atomic orbital energies, the 1s orbital of hydrogen and the 3s orbital of sulfur interact only weakly; this is shown in the diagram by a slight stabilization of
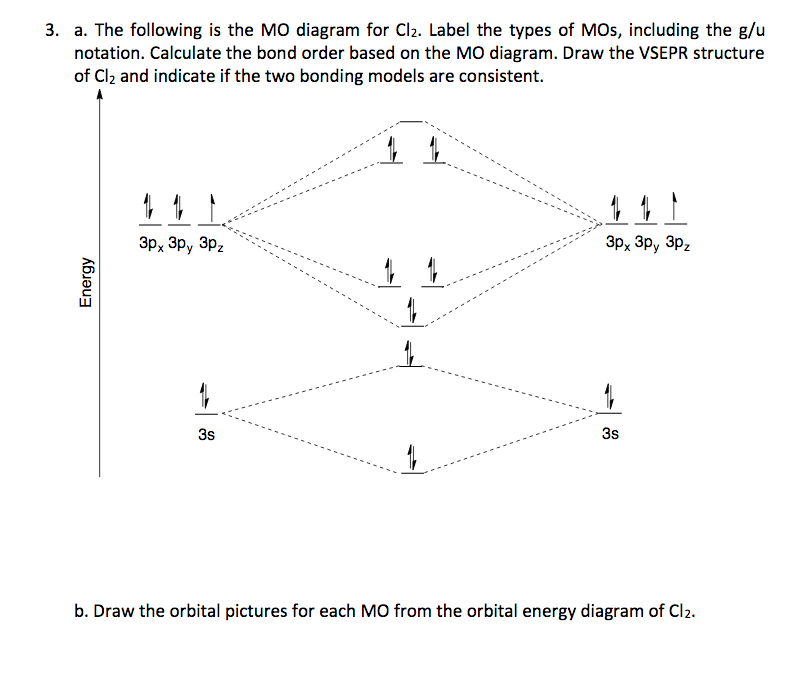
5) Draw the molecular orbital diagram for Cl2. Assume 3s and 3p orbitals show similar LCAO as 2s and 2p orbitals (Label increasing energy using an arrow next to the diagram. Label each atomic and each molecular orbital) Calculate the bond order and give the molecular orbital configuration for the valence electrons.
Nonbonding Molecular Orbitals — When we draw a molecular orbital diagram for a molecule, there are four key points to remember: The number of molecular ...
4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
Problem: Draw a molecular orbital diagram for Ar2+. This ion has been observed in the gas phase. Calculate bond order and describe how the bond distance in this ion would differ from that in Cl2. FREE Expert Solution. 87% (383 ratings) Problem Details.
Cl2 Molecular Orbital Diagram. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals.
Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Chronic (long-term) exposure to chlorine gas in workers has resulted in respiratory effects, including eye and throat irritation and airflow obstruction. No information is available on the carcinogenic effects of chlorine in humans from inhalation ...
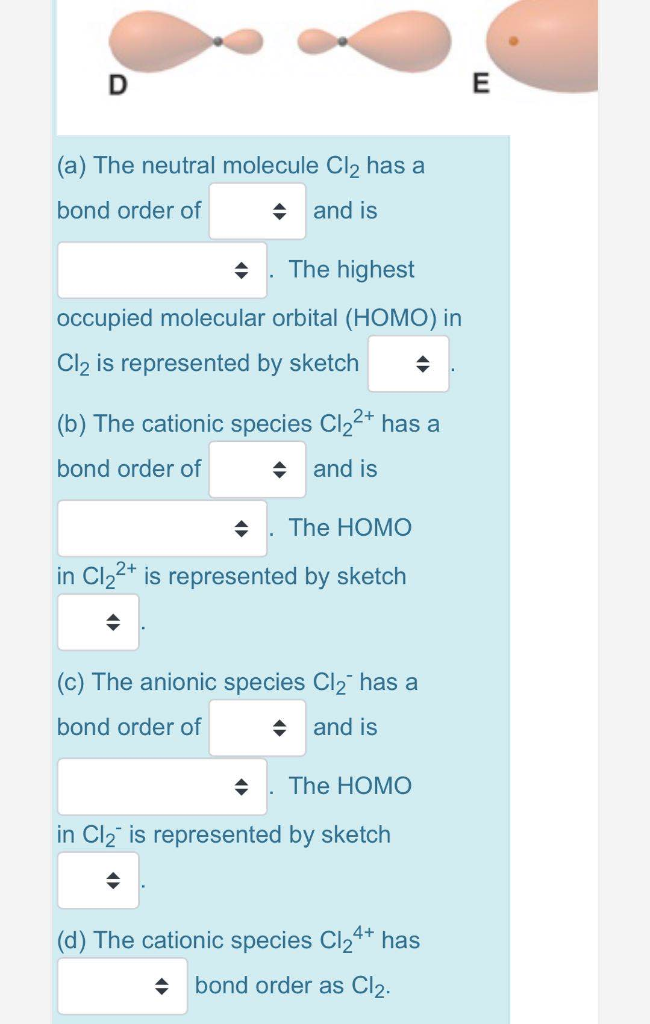
We're being asked to determine the bond order of Cl2+. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: The molecular orbital diagram for Cl2 ...
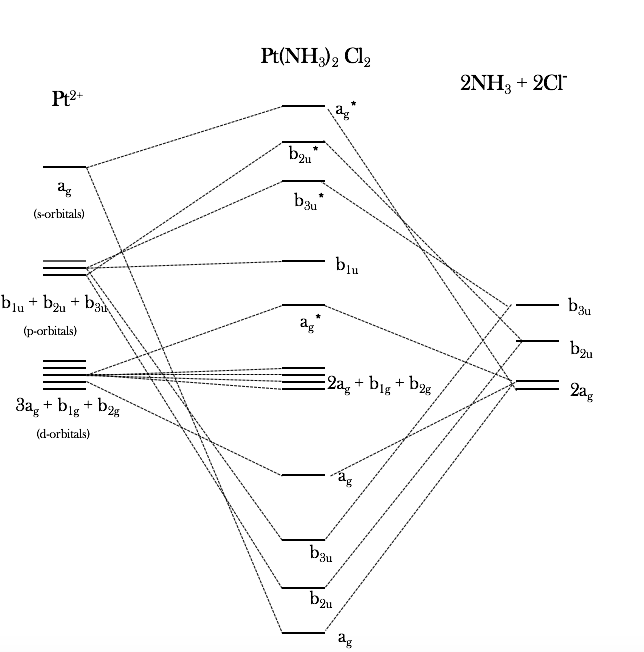
Transcribed image text: 5. Draw a molecular orbital diagram for a sigma bonded complex ion of [Ni(NH3).]Cl2 [6] (i) Calculate the Ao, then determine if it is low- or high-spin [2] (ii) Which metal orbitals are utilized in forming molecular orbitals [3] (iii) How many molecular orbitals will result from the overlap of the LGO's (ligand group orbitals) with the metal orbitals?
Bond order = 1/2[Nb-Na], where, Nb=no. of electrons in bonding molecular orbital and Na= no. of electrons in anti-bonding molecular orbital. So bond order = (9– ...2 answers · 16 votes: B.O. of Cl2 = 1
This is the molecular orbital diagram for the homonuclear diatomic Be2+, showing the molecular orbitals of the valence shell only. The molecular orbitals are. Watch the video solution for the question: Consider the species Cl2+, Cl2, and Cl2-. Wh Consider the molecular orbital diagram for oxygen, Consider the.
Question: (a) Draw a molecular orbital energy diagram for Cl2 and show which orbitals are occupied with electrons. (b) How many bands are expected in the ...
Answer (1 of 2): B.O. of Cl2 = 1
According to Molecular Orbital Theory, its electronic configuration is as follows: $1s2 $*1s2. CH4 + 2 Cl2--> CH2Cl2 + 2HCl Cl-Cl bond energy: 242 kj/mole 1. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. Identify HOMO and LUMO.
Molecular Orbital Diagrams. An MO diagram, just like an atomic orbital diagram, shows the relative energy and number of electrons in each MO. The MO diagram also shows the AOs from which each MO is formed. Bond order is calculated as follows: ½[(# of e-in bonding MO) - (# of e-in antibonding MO)]

A Draw A Molecular Orbital Energy Diagram For Cl2 And Show Which Orbitals Are Occupied With Electrons B How Many Ba Homeworklib

Use The Drawing Of Mo Energy Diagram To Predict The Bond Order Of L I 2 And L I 2 1 Do You Expect L I 2 To Exist








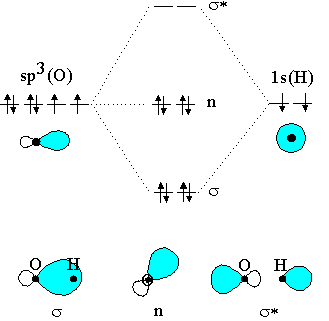






0 Response to "40 molecular orbital diagram for cl2"
Post a Comment