45 potential energy diagram exothermic
Answered: Draw the potential energy diagram of an… | bartleby Draw the potential energy diagram of an exothermic reaction. Illustrate, using two-way arrows, the items below. Give a title to each of the axes and to the diagram, and indicates the energy level of the components of the reaction. The change in enthalpy The activation energy of the direct reaction The energy of the reverse reaction What is a potential energy diagram? - Moorejustinmusic What is a potential energy diagram? A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. Potential energy diagrams for endothermic and exothermic reactions are described. How do you define kinetic and potential energy?
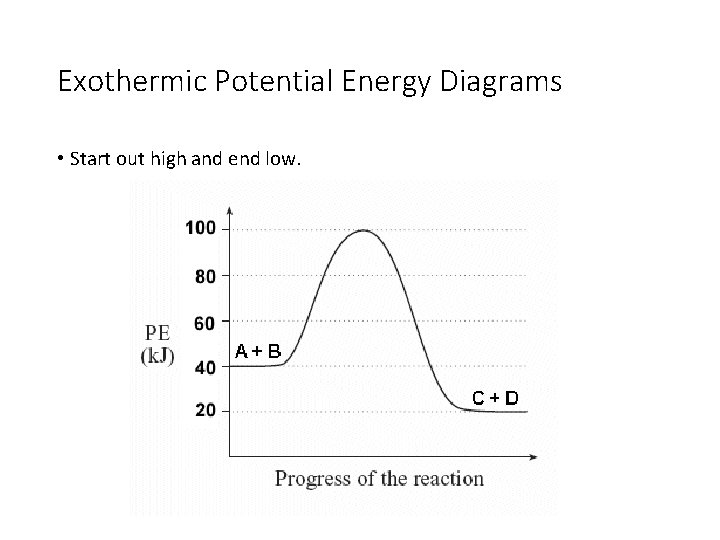
How to draw the potential energy diagram for this reaction ... 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
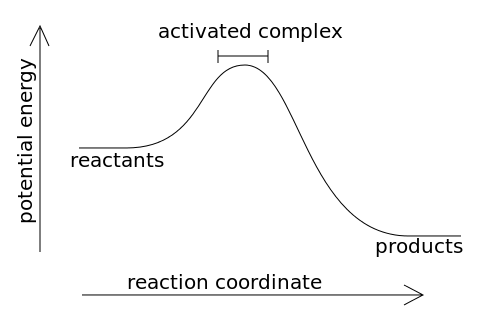
Potential energy diagram exothermic
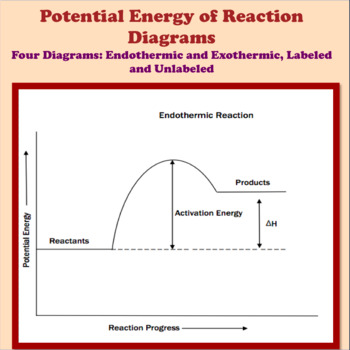
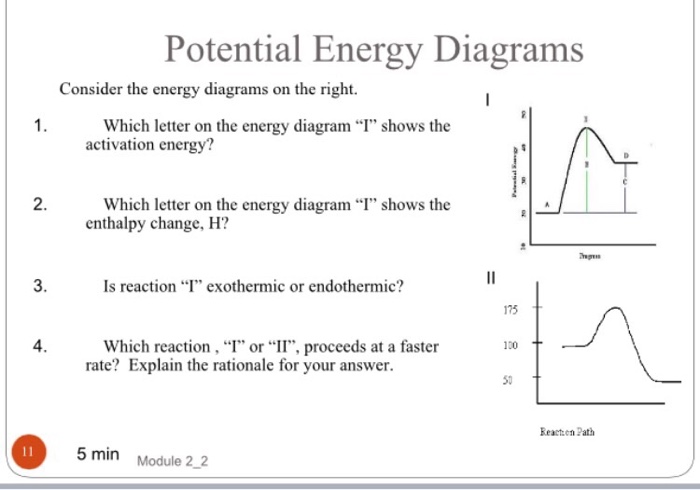
Potential Energy Diagrams ( Read ) | Chemistry | CK-12 ... Graphs of the energy changes that occur during a chemical reaction. Estimated5 minsto complete. %. Progress. Practice Potential Energy Diagrams. MEMORY METER. This indicates how strong in your memory this concept is. Practice. Preview. Potential Energy Diagrams - Kentchemistry.com Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products. Potential Energy Diagram Practice Endothermic and ... Students will be given two Potential Energy Diagrams -- one Endothermic, one Exothermic. They will have to read the graph and determine if the graph is showing an endothermic or exothermic reaction, justify why it is so, then calculate the heat of reaction (delta H), activation energy (Ea), reverse activation energy, answer four conceptual multiple choice questions, and then determine if 9 ...
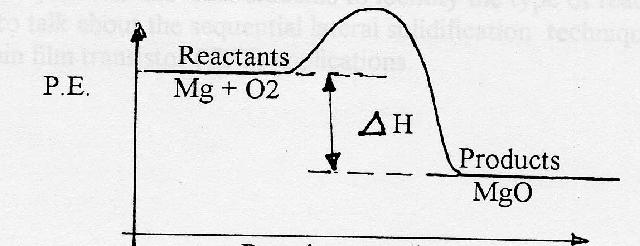
Potential energy diagram exothermic. PDF Potential Energy Diagram Worksheet - games.ohio.com Exothermic Energy Diagram: Activation Energy, Transition States and Enthalpy Change - TUTOR HOTLINE SN1 Reaction Energy Diagram Draw a Potential Energy Curve for this Reaction (Given Mechanism) FSc Chemistry Book1, CH 11, LEC 13: Potential Energy Diagram for Reaction Mechanism Potential Energy Diagram Calculations (Level 2) 25.3 Reading ... Potential Energy Diagrams - Chemistry - Catalyst ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... How does the energy level diagram show this reaction is ... Label ΔH as positive or negative. Figure shows the energy level diagram for the reaction between methane and oxygen. Based on Figure, the following information can be obtained. (a) The reaction between methane and oxygen to form carbon dioxide and water is an exothermic reaction. (b) During the reaction, the temperature of the mixture increases. Endothermic and Exothermic Reactions With Potential Energy ... This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
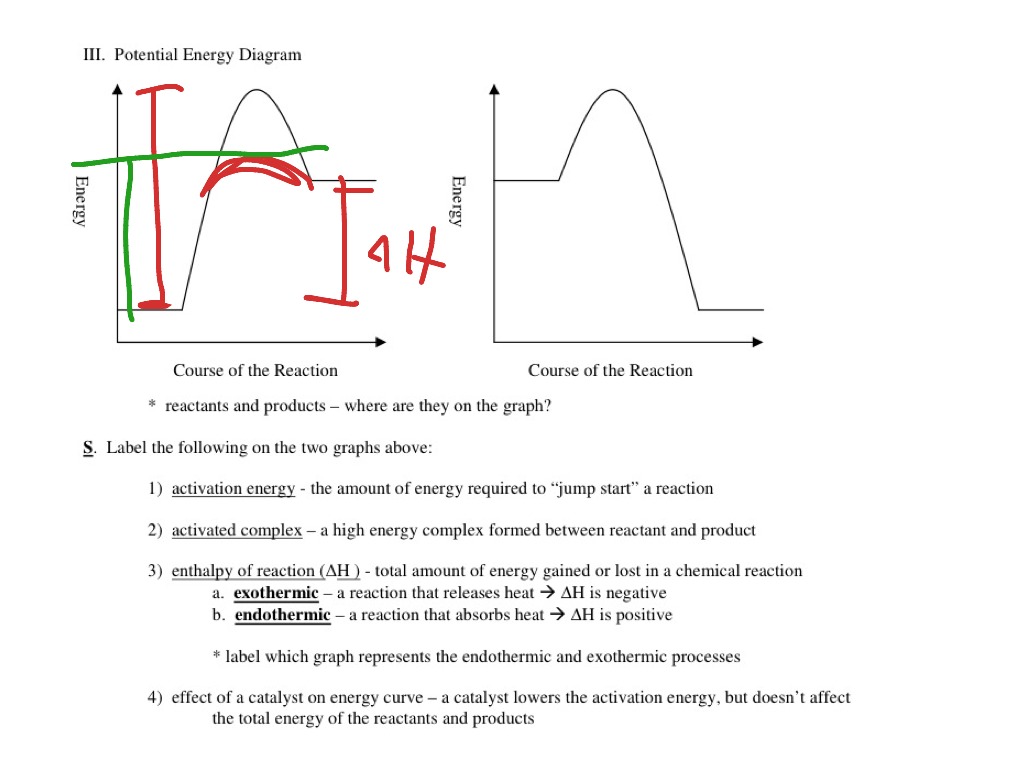
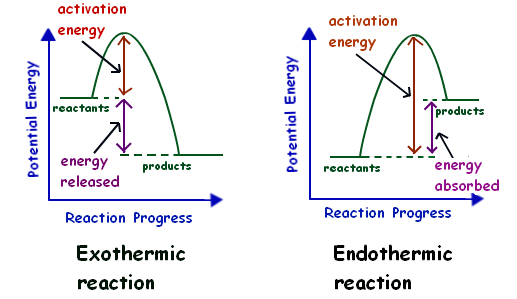
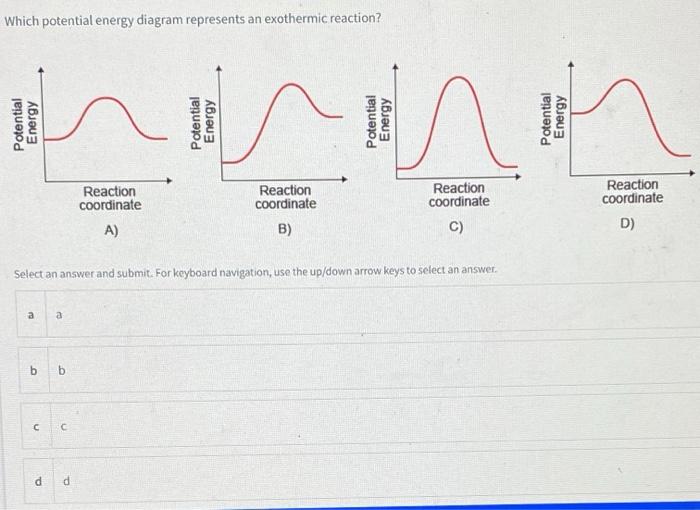
Draw the potential energy diagram for an exothermic ... Draw the potential energy diagram for an exothermic reaction. Explain the terms: activation energy of backward reaction. Updated On: 21-7-2021. This browser does not support the video element. Answer. Step by step solution by experts to help you in doubt clearance & scoring excellent marks in exams. Solved Which potential energy diagram represents an ... Question: Which potential energy diagram represents an exothermic reaction? Potential Energy Potential Energy non Reaction coordinate A) Reaction coordinate B) Reaction coordinate C) Reaction coordinate D) Select an answer and submit. For keyboard navigation, use the up/down arrow keys to select an answer. a a b b с c d d Which potential ... PDF Potential Energy Diagrams potential energy diagrams are a visual representation of potential energy in a chemical reaction note the x axis is usually reaction cordinate or time ... exothermic (loss of energy) if reactants have less energy then products, the reaction is endothermic (gain of energy) Representing endothermic and exothermic processes using ... Representing endothermic and exothermic processes using energy diagrams AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
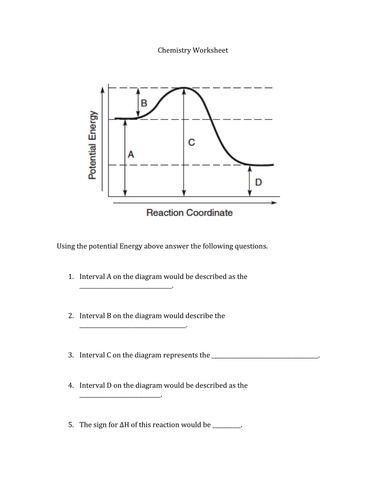
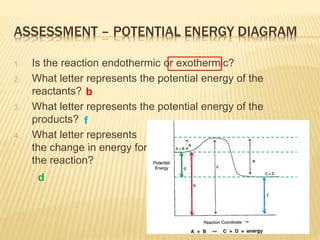
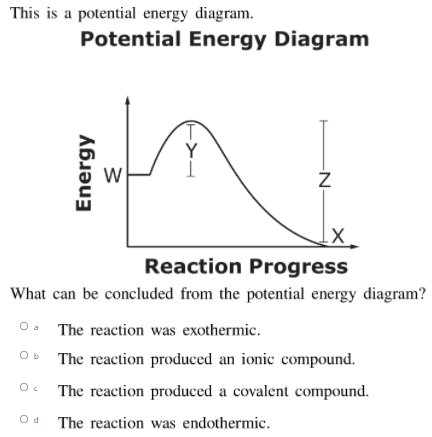
How do you know if a potential energy diagram is ... Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. The total potential energy of the system increases for the endothermic reaction as the system absorbs energy from the surroundings. What is the order of E1 elimination reaction? ws_potential_energy_diagram_answers.pdf - Potential Energy ... Complete the potential energy diagram for an exothermic reaction, where the reactants have 250 kJ of potential energy stored in their chemical bonds, there is 100 kJ of activation energy required and the products have 50 kJ of potential energy stored in their chemical bonds. Scale and label the y-axis for kJ of potential energy. Solved Select the potential energy diagram that represents ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Exothermic reactions are characterized by negative change in …. View the full answer. Transcribed image text: Select the potential energy diagram that represents an exothermic (exergonic) reaction. Potential Energy Diagram Worksheet - Jojo Worksheet Potential energy diagram worksheet. 150 100 Potential Energy X2Y2 50 Progress of Reaction 1. Write the formula for determining ΔH 2. Label the axis PE of reactants 350 KJmol Ea 100 KJmol PE of products 250 KJmol. There are a number of important qualitative features of the behavior of the system that can be determined by just knowing this curve.
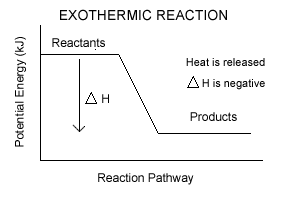
Energy Diagrams of Reactions | Fiveable In the graph for an exothermic reaction, you can see that the products have a lower potential energy, implying that energy has been released from the system. This further proves that ΔH is negative in an exothermic reaction. Image Courtesy of SilaVula Endothermic Reactions
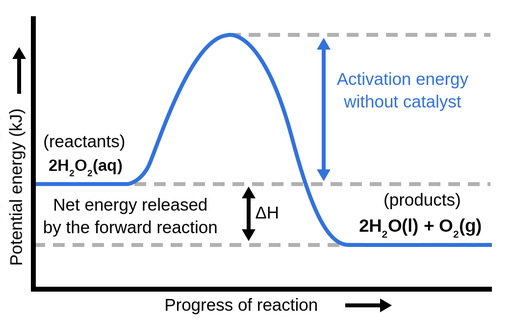
Endothermic vs Exothermic and Energy Diagrams.pdf - Name ... EXOTHERMIC Label your diagram - if you are filling in electronically, click on diagram, click "edit" and fill in correct words in text boxes already provided. Key things to Note about EXOTHERMIC Energy Diagram: Reactants have MORE potential energy compared to Products Energy was released to the environment during the reaction (this is why products have less energy) Δ H will be negative ...
Potential Energy Diagrams | Chemistry for Non-Majors A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction.
Potential energy diagrams - Controlling the rate - Higher ... Potential energy diagrams can be used to calculate both the enthalpy change and the activation energy for a reaction. Exothermic reactions An exothermic reaction is one in which heat energy is...
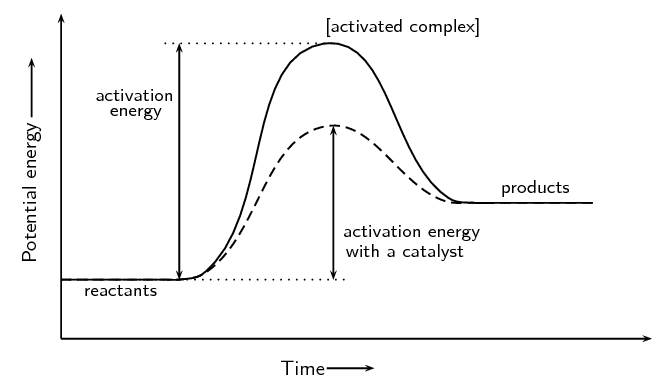
Potential energy diagram with/without catalyst in a ... Potential energy diagram with/without catalyst in a hypothetical exothermic chemical reaction coordinate of Boltzmann distribution. The presence of the catalyst opens a different reaction pathway ...
Endothermic vs. exothermic reactions (article) | Khan Academy Phase diagrams. Enthalpy. Heat of formation. Hess's law and reaction enthalpy change. Gibbs free energy and spontaneity. Gibbs free energy example. More rigorous Gibbs free energy / spontaneity relationship. A look at a seductive but wrong Gibbs spontaneity proof. Endothermic vs. exothermic reactions.
What statement describes the potential energy diagram of ... How will an energy diagram look for an exothermic reaction? Moving from left to right, the curve will end up lower than where it started. This is because in an exothermic reaction, the potential ...
Smart Exothermic Potential Energy Diagram Is There An ... Potential energy diagrams chemistry catalyst endothermic exothermic reactions duration. The reaction in question is exothermic releases heat hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
Potential Energy Diagram Worksheets & Teaching Resources | TpT Students will be given two Potential Energy Diagrams -- one Endothermic, one Exothermic. They will have to read the graph and determine if the graph is showing an endothermic or exothermic reaction, justify why it is so, then calculate the heat of reaction (delta H), activation energy (Ea), reverse
Potential Energy Diagram Worksheet - Worksheet Smart Potential energy diagram worksheet 1. Draw a line segment that represents the potential energy of the reactants and label the line. Draw an energy diagram for a reaction. Nat is tne cnmatpy change ah tor the d what is the enthalpv ch forward reaction. Is the overall reaction as shown exothermic or endothermic.
Potential Energy Diagram Practice Endothermic and ... Students will be given two Potential Energy Diagrams -- one Endothermic, one Exothermic. They will have to read the graph and determine if the graph is showing an endothermic or exothermic reaction, justify why it is so, then calculate the heat of reaction (delta H), activation energy (Ea), reverse activation energy, answer four conceptual multiple choice questions, and then determine if 9 ...
Potential Energy Diagrams - Kentchemistry.com Both endothermic and exothermic reactions require activation energy. Activated complex In this diagram, the activation energy is signified by the hump in the reaction pathway and is labeled. At the peak of the activation energy hump, the reactants are in the transition state, halfway between being reactants and forming products.
Potential Energy Diagrams ( Read ) | Chemistry | CK-12 ... Graphs of the energy changes that occur during a chemical reaction. Estimated5 minsto complete. %. Progress. Practice Potential Energy Diagrams. MEMORY METER. This indicates how strong in your memory this concept is. Practice. Preview.























0 Response to "45 potential energy diagram exothermic"
Post a Comment