45 orbital diagram for zinc
What is the orbital notation for zinc? - Answers What is the orbital notation for zinc? - Answers 1s with two arrows, 2s with 2 arrows, 2p with 6 arrows, 3s with 2 arrows, 3p with 6 arrows, 4s with 2 arrows, 3d with 10 arrow; Remember that... Orbital Diagram For Selenium - schematron.org In writing an. Answer to orbital diagram for selenium home / study / science / chemistry / chemistry questions and answers / Orbital Diagram For Selenium. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4.Selenium (Se) has an atomic mass of Find out about its chemical and physical properties, states, energy ...
Copper(Cu) electron configuration and orbital diagram Therefore, an electron of 4s orbital completes a full-filled 3d orbital by jumping into a 3d orbital. So, the copper(Cu) electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 1. How to write the orbital diagram for copper(Cu)? To create an orbital diagram of an atom, you first need to know Hund's principle and Pauli's exclusion ...

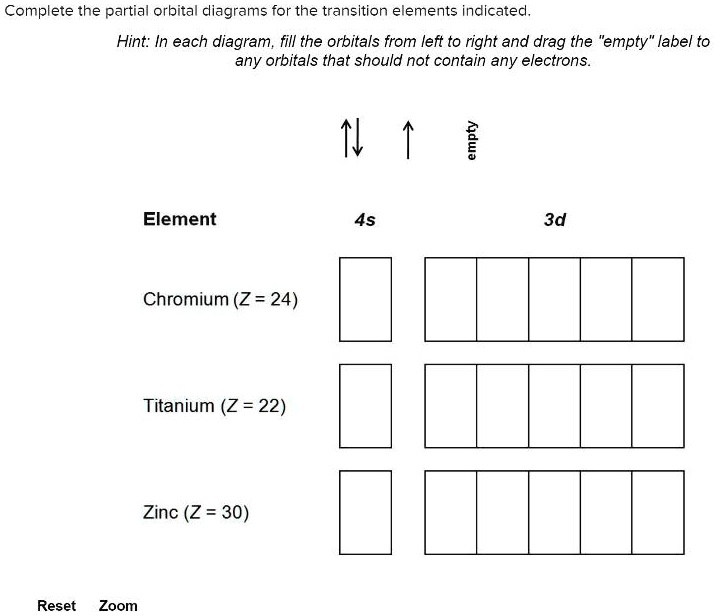
Orbital diagram for zinc
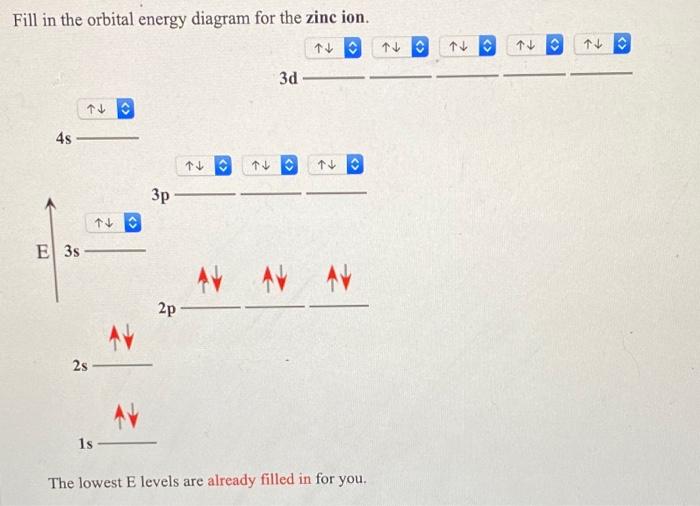
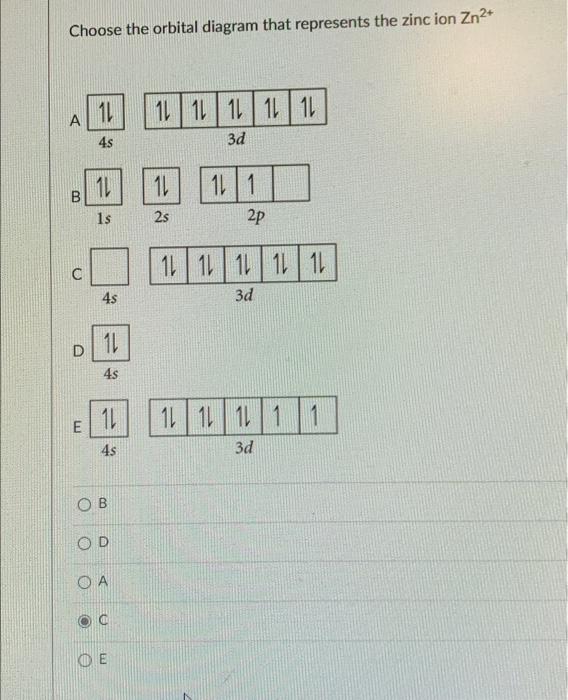
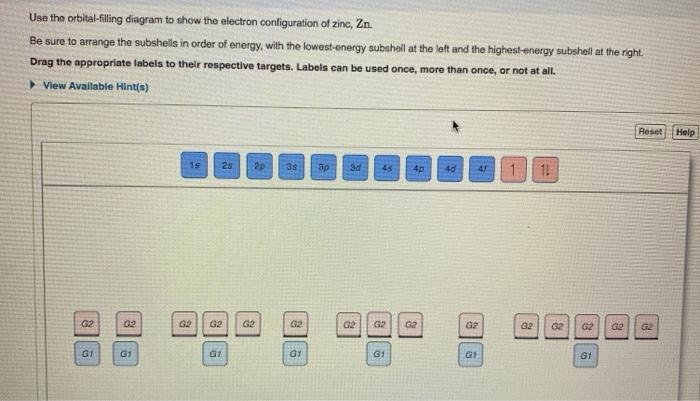
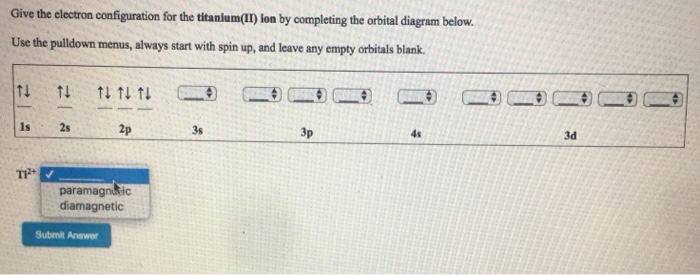
What is the electron configuration for Zn2+? | Socratic Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has higher energy than the d-sublevel, which is contrary to what the Aufbau diagram indicates. When d-block elements lose electrons, they lose the highest energy s electrons first, which in the case of zinc are the two 4s electrons. Solved Fill in the orbital energy diagram for the zinc ion ... Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (4 ratings) Transcribed image text: Fill in the orbital energy diagram for the zinc ion The lowest E levels are already filled in for you. Chem4Kids.com: Zinc: Orbital and Bonding Info Zinc atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ...
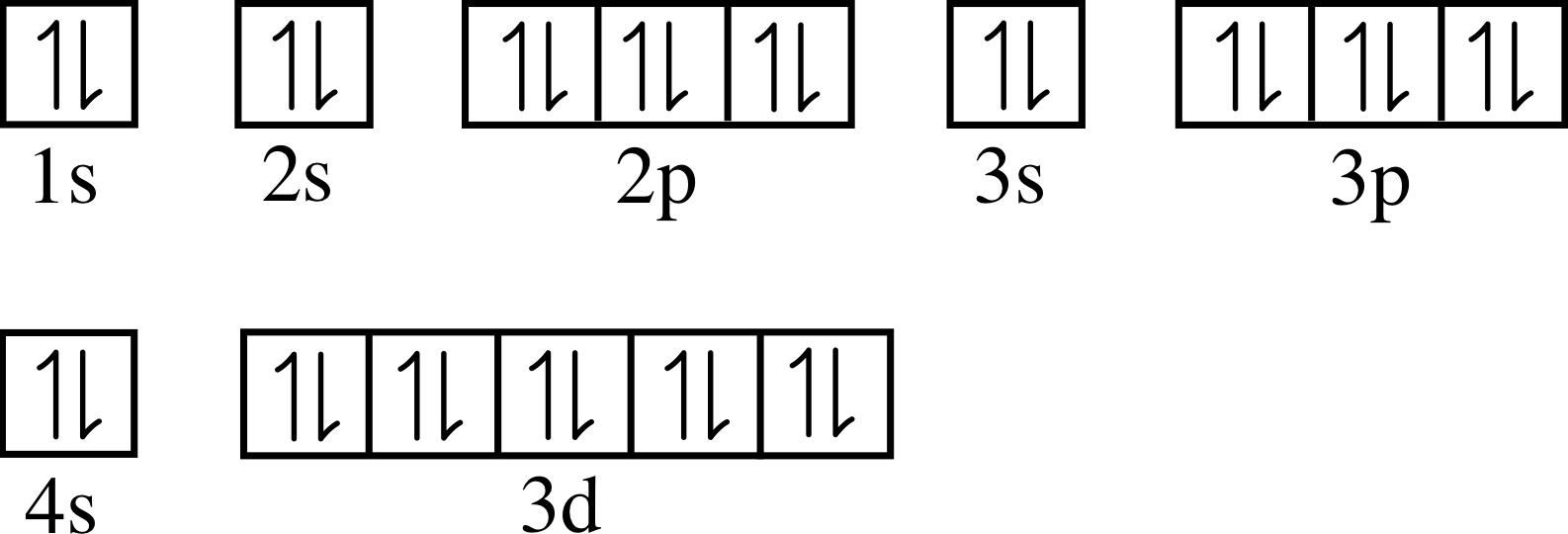
Orbital diagram for zinc. Electron configuration for Zinc (element 30). Orbital diagram Zn (Zinc) is an element with position number 30 in the periodic table. Located in the IV period. Melting point: 419.6 ℃. Density: 7.14 g/cm 3 . Electronic configuration of the Zinc atom in ascending order of orbital energies: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Choose The Valence Orbital Diagram That Represents The ... Choose the valence orbital diagram that represents the ground state of zn. Choose the valence orbital diagram that represents the ground state of se2⁻. One electron can jump from a lower to a higher energy orbital, thus creating unpaired electrons. A partial orbital diagram shows only the highest energy sublevels being filled. Number of electrons, electron configuration, orbital ... Solution for Number of electrons, electron configuration, orbital diagram, and magnetic property of zinc. close. Start your trial now! First week only $4.99! arrow_forward. learn. write. tutor. study resourcesexpand_more. Study Resources. We've got the study and writing resources you need for your assignments. ... en.wikipedia.org › wiki › Diels–Alder_reactionDiels–Alder reaction - Wikipedia (The same conclusion can be drawn from an orbital correlation diagram or a Dewar-Zimmerman analysis.) For the more common "normal" electron demand Diels–Alder reaction, the more important of the two HOMO/LUMO interactions is that between the electron-rich diene's ψ 2 as the highest occupied molecular orbital (HOMO) with the electron ...
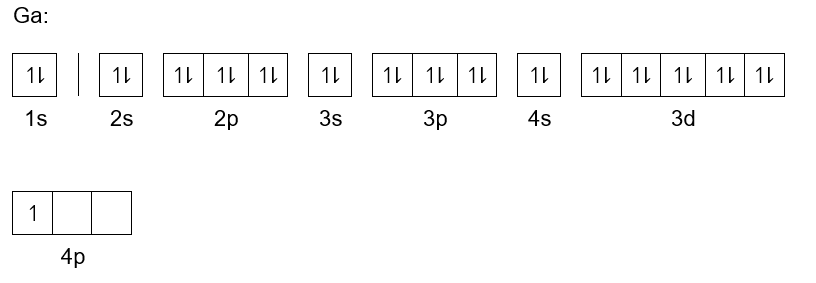
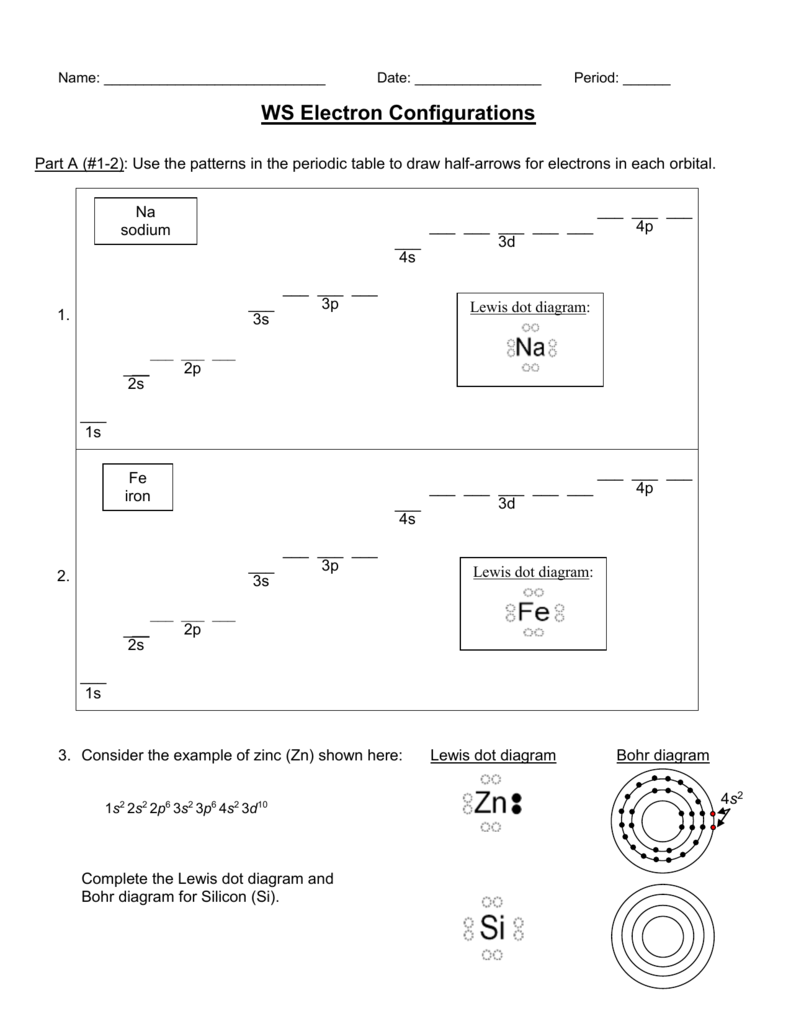
Arrangements of electrons in the orbitals of an atom is ... Zinc has an electron configuration of [Ar]4s 2 3d 10. At gallium we begin filling the 4p sublevel and continue to krypton. Rubidium fills the 5s, yttrium the 4d and indium the 5p. Cesium fills the 6s and lanthanum bigins the first available f sublevel, the 4f. The f sublevel is filled from lanthanum through ytterbium. Zinc atomic orbital diagram example. - YouTube Zinc. Atomic Orbital. Electron orientation PDF Electron Configurations and Orbital Diagrams key 1. Describe the two differences between a 2p x orbital and a 3p y orbital. The 2px orbital lies on the x-axis. The 3py orbital lies on the y-axis and is larger than the 2px orbital. 2. The lobes of a p orbital disappear at the nucleus. What does this tell us about electrons in p orbitals? PDF Orbital diagram for zinc ion - static.s123-cdn-static.com Orbital diagram for zinc ion The purpose of the introduction of quantum numbers is to show that similarities in the electron control or electron configuration lead to the similarities and differences in the properties of elements.
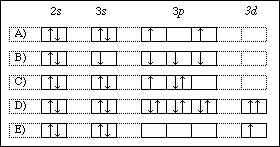
study.com › learn › electron-configuration-questionsElectron Configuration Questions and Answers | Study.com Using an orbital box diagram and noble gas notation, show the electron configuration of the gadolinium(III) ion. ... Write the full electron configuration for zinc (Z = 30). Orbital Diagram of Zinc (Zn), Electron Configuration, and ... Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom. zinc orbital diagram - Pastor Choolwe Write an orbital diagram for the ground state of the zinc atom. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. Write an orbital diagram for the ground state of the zinc ... What is the ground state electron configuration and orbital diagram of Os(76)? Is this paramagnetic or diamagnetic? (b) The following is an excited state configuration of a neutral atom: 1s22s22p53s1 3p44s2 5s26s15p5 Identify the element and write its ground state electronic configuration.
Mo3+ Orbital Diagram - Wiring Diagrams Mo3+ Orbital Diagram. Compact version of orbital energy diagram with each orbital represented . Mo2+, Mo3+, Mo4+ and Mo5+ are all known in aqueous solution. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital.
› publication › 337797765_A(PDF) A Textbook of Physical Chemistry – Volume 1 An advanced-level textbook of physical chemistry for the graduate (B.Sc) and postgraduate (M.Sc) students of Indian and foreign universities. This book is a part of four volume series, entitled "A ...
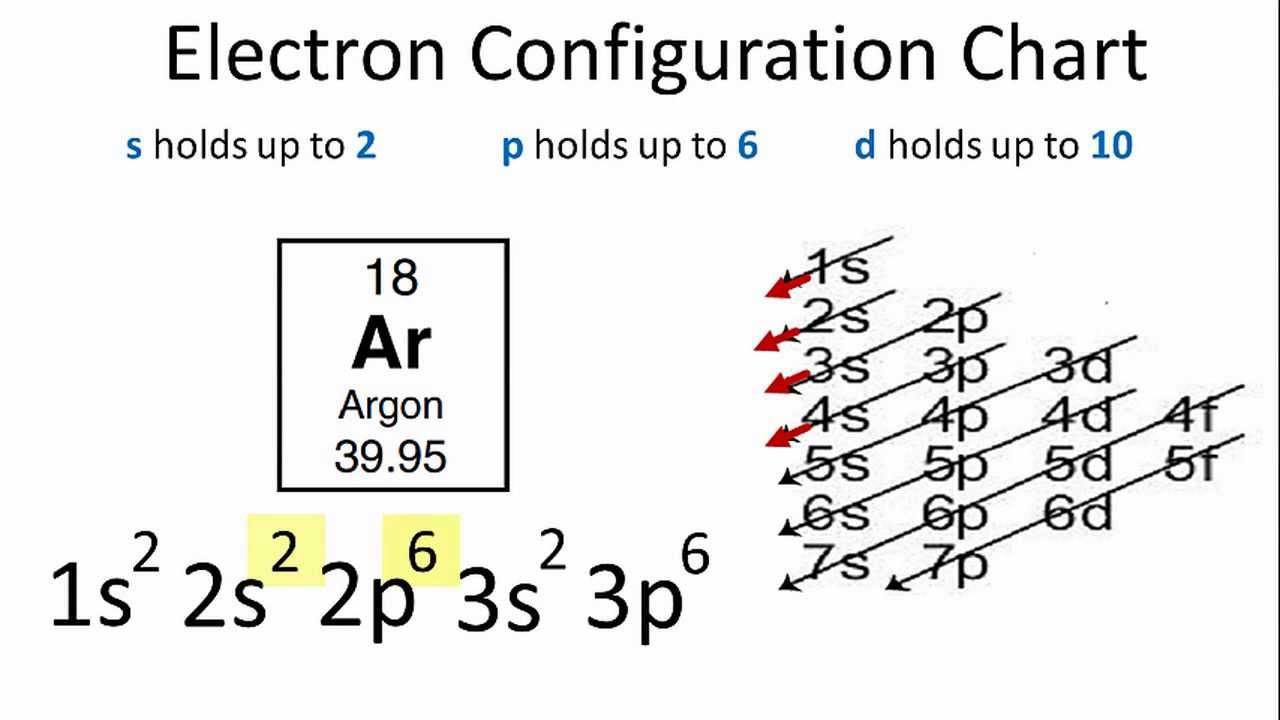
Zinc(Zn) electron configuration and orbital diagram Zinc (Zn) orbital diagram 1s is the closest and lowest energy orbital to the nucleus. Therefore, the electron will first enter the 1s orbital. According to Hund's principle, the first electron will enter in the clockwise direction and the next electron will enter the 1s orbital in the anti-clockwise direction.
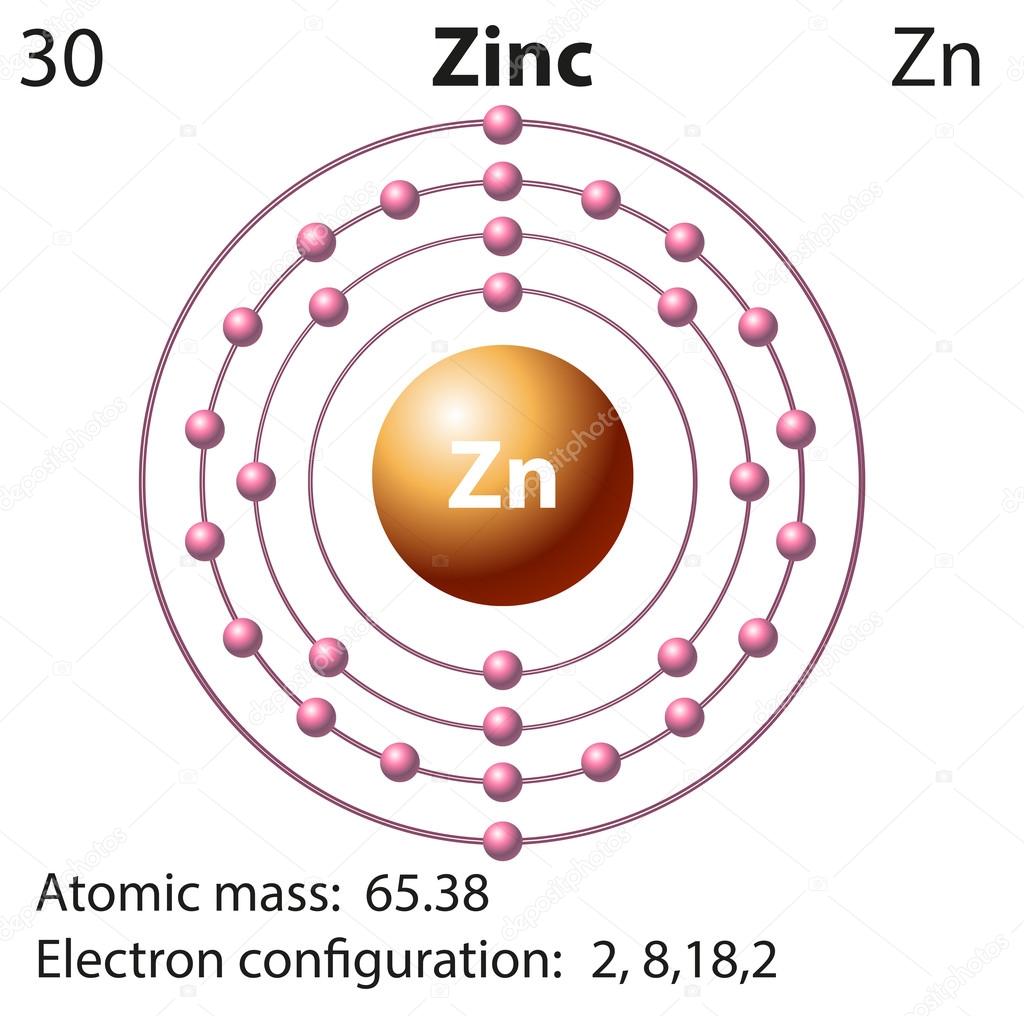
Zinc, atomic structure - Stock Image - C018/3711 - Science ... Zinc (Zn). Diagram of the nuclear composition, electron configuration, chemical data, and valence orbitals of an atom of zinc-65 (atomic number: 30), an isotope of this element. The nucleus consists of 30 protons (red) and 35 neutrons (orange). 30 electrons (white) successively occupy available electron shells (rings).
What is the orbital diagram for zinc? - AnswersToAll What is the orbital diagram for zinc? Electrons & Oxidation Why Valency of zinc is 2? Zinc is a d-Block element and belongs to Transition Metal. Valence shell contains 2 electrons that is 4s2 that means that zinc can lose the two electrons located in the 4s-orbital to be become the Zn2+ cation. So, the valency of Zinc is 2.
opentextbc.ca › chapter › 8-2-hybrid-atomic-orbitals8.2 Hybrid Atomic Orbitals – Chemistry This orbital energy-level diagram shows the sp hybridized orbitals on Be in the linear BeCl 2 molecule. Each of the two sp hybrid orbitals holds one electron and is thus half filled and available for bonding via overlap with a Cl 3 p orbital.
Orbital Diagram of Zinc (Zn), Electron Configuration, and ... Jun 6, 2018 - Orbital Diagram, electron configuration, and the noble gas notation for a zinc (Zn) atom.
chemguide.co.uk › inorganic › complexionscomplex ions - colour - chemguide Zinc with the electronic structure [Ar] 3d 10 4s 2 doesn't count as a transition metal whichever definition you use. In the metal, it has a full 3d level. In the metal, it has a full 3d level. When it forms an ion, the 4s electrons are lost - again leaving a completely full 3d level.
en.wikipedia.org › wiki › Saturn_V_Instrument_UnitSaturn V instrument unit - Wikipedia The Saturn V instrument unit is a ring-shaped structure fitted to the top of the Saturn V rocket's third stage and the Saturn IB's second stage (also an S-IVB). It was immediately below the SLA (Spacecraft/Lunar Module Adapter) panels that contained the Apollo Lunar Module.
The orbital diagram for the ground state of Zinc and its ... The orbital diagram for the ground state of Zinc and its magnetic nature has to be written. Concept introduction: Pauli Exclusion Principle An orbital having a most two electrons and in this two electrons have opposite spins. Each orbital having no more than two electrons and similar spin is not allowed. Aufbau principle
WebElements Periodic Table » Zinc » properties of free atoms Electron binding energies for zinc. All values of electron binding energies are given in eV. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules; relative to the Fermi level for metals; and relative to the top of the valence band for semiconductors. Label Orbital eV [literature ...
What is the electron dot diagram for zinc class 12 ... Hint: Electron dot diagram also known as Lewis diagram is a way to represent the number of electrons present in the valence shell of an element.In the diagram, the number of dots on the symbol of the element represent its valence electrons. Complete answer: To find the electron dot diagram for zinc, we need to write its electronic configuration according to Aufbau's Principle.
Solved Fill in the orbital energy diagram for the zinc ion ... Science. Chemistry. Chemistry questions and answers. Fill in the orbital energy diagram for the zinc ion 3d 45 3p E 35 AV NV 2p NV 25 AV ls.
valenceelectrons.com › chlorine-electron-configurationChlorine(Cl) electron configuration and orbital diagram Orbital diagram for chlorine(Cl) Chlorine(Cl) excited state electron configuration. Atoms can jump from one orbital to another orbital by excited state. This is called quantum jump. Ground state electron configuration of chlorine is 1s 2 2s 2 2p 6 3s 2 3p 5. The valency of the element is determined by electron configuration in the excited state.
Mo3+ Orbital Diagram - schematron.org Mo3+ Orbital Diagram. That's why Mo3+ has 39 electrons instead of 42 in its ground state electron The normal electron configuration of zinc is [Ar] 3d 10 4s 2, with 2 4s orbital. However, even though the 5s orbital is lower in energy than the 4d orbital, the electrons in the 4d orbitals shield the electron in the 5s orbitals.
PDF Orbital diagram for zinc - static.s123-cdn-static.com Write an orbital diagram for the state of the earth of the zinc atom. The molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonds in molecules in terms of molecular orbital theory in general and in particular the linear combination of atomic orbital methods (LCAO).
Orbital Diagram of All Elements (Diagrams given Inside) Orbital diagram of Zinc (Zn) 31: Orbital diagram of Gallium (Ga) 32: Orbital diagram of Germanium (Ge) 33: Orbital diagram of Arsenic (As) 34: Orbital diagram of Selenium (Se) 35: Orbital diagram of Bromine (Br) 36: Orbital diagram of Krypton (Kr) 37: Orbital diagram of Rubidium (Rb) 38: Orbital diagram of Strontium (Sr) 39:
Chem4Kids.com: Zinc: Orbital and Bonding Info Zinc atomic orbital and chemical bonding information. There are also tutorials on the first thirty-six elements of the periodic table. Check out the blackboard. That box on the left has all of the information you need to know about one element. It tells you the mass of one atom, how many ...
Solved Fill in the orbital energy diagram for the zinc ion ... Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (4 ratings) Transcribed image text: Fill in the orbital energy diagram for the zinc ion The lowest E levels are already filled in for you.
What is the electron configuration for Zn2+? | Socratic Zinc is a d-block element, also known as a transition element. For the d-block elements, the outermost s-sublevel has higher energy than the d-sublevel, which is contrary to what the Aufbau diagram indicates. When d-block elements lose electrons, they lose the highest energy s electrons first, which in the case of zinc are the two 4s electrons.
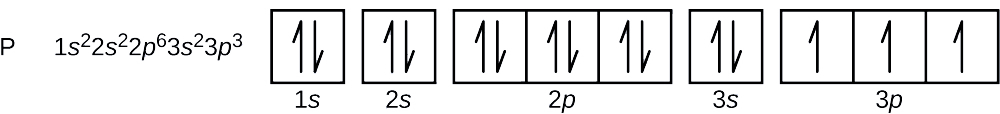




























0 Response to "45 orbital diagram for zinc"
Post a Comment