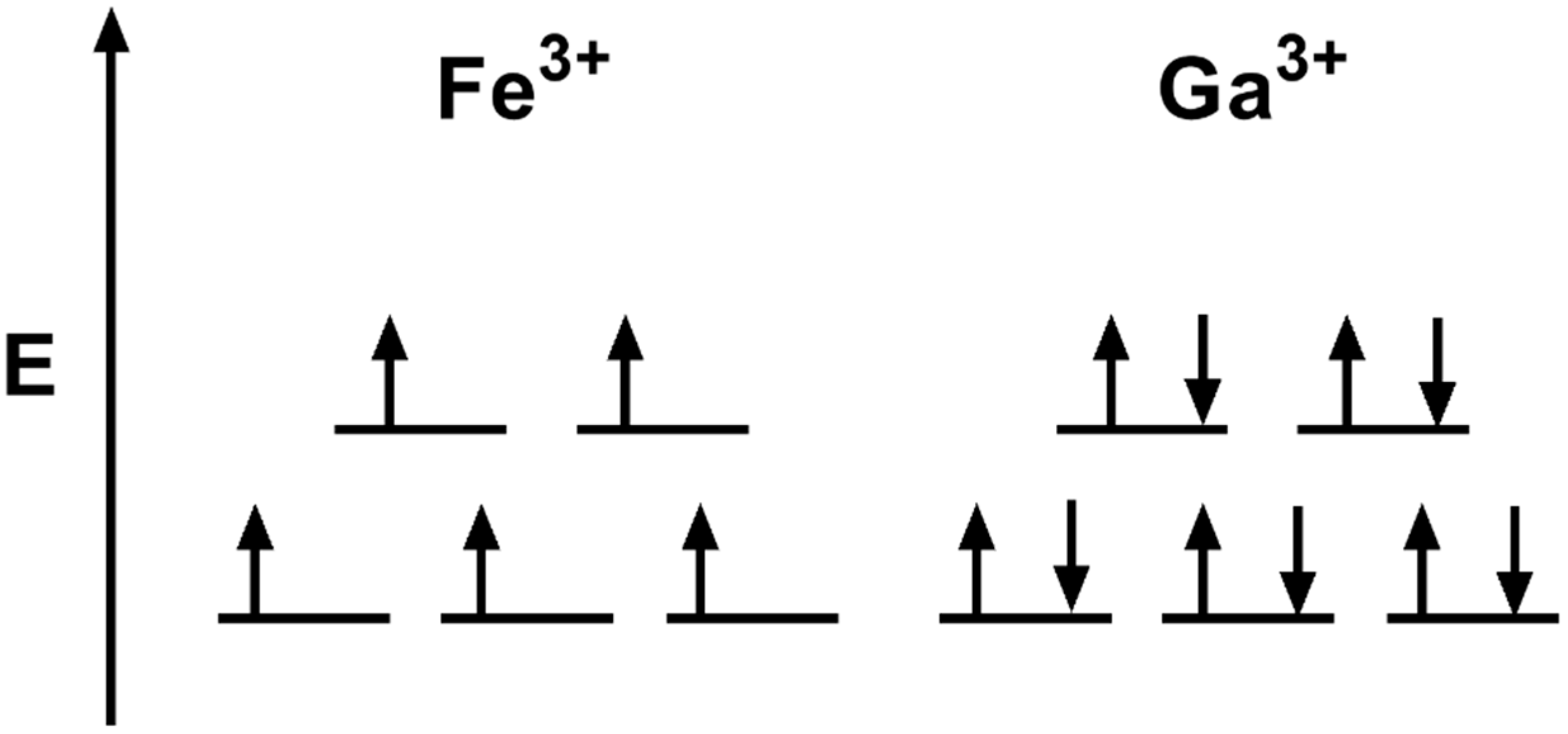
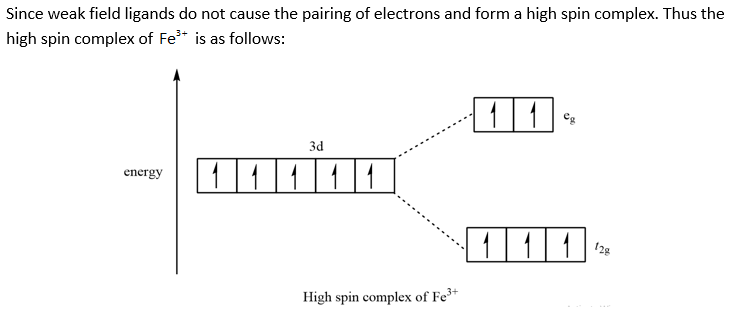
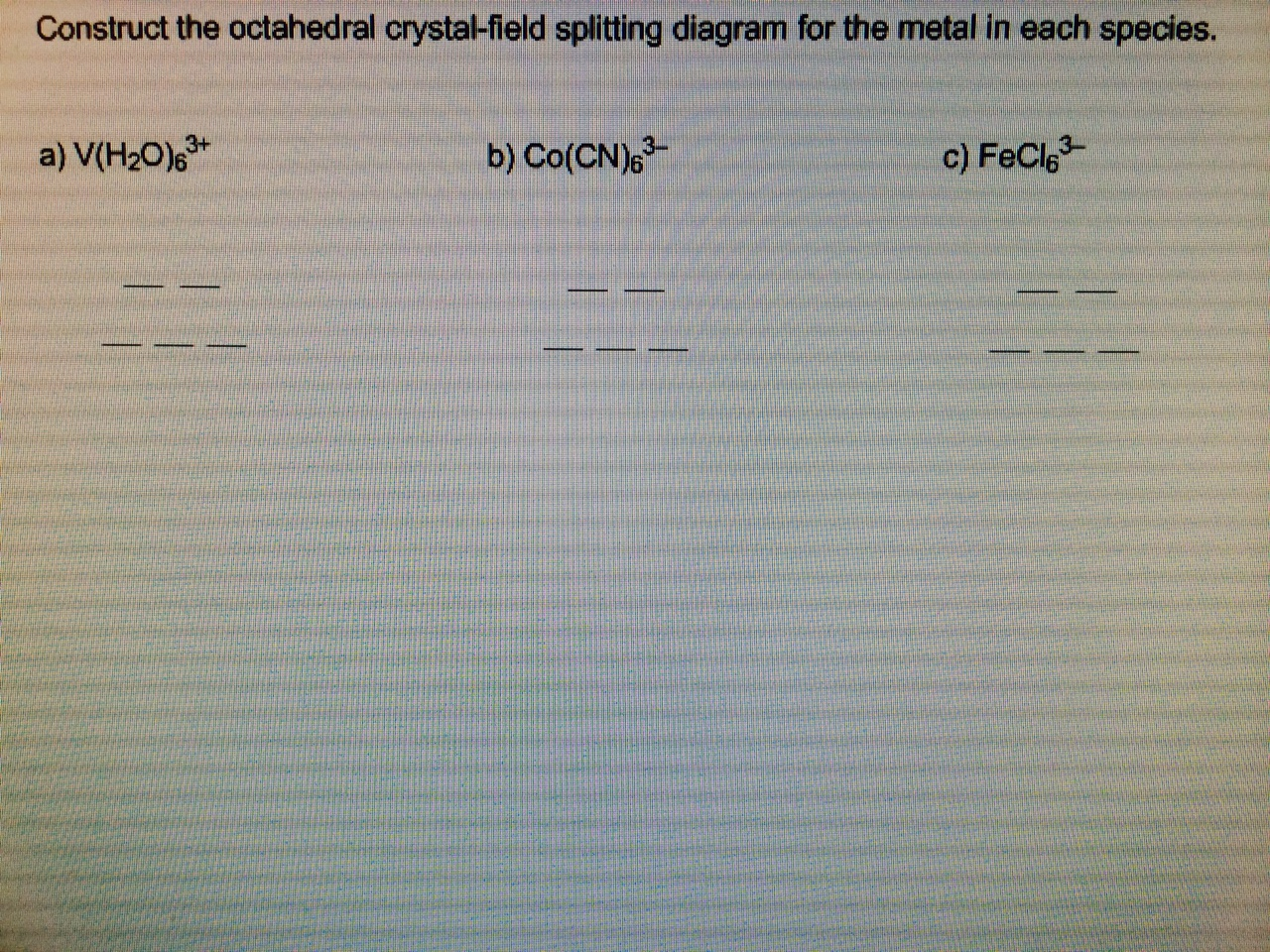
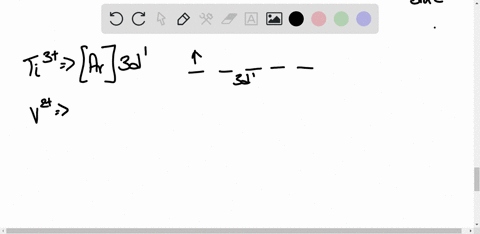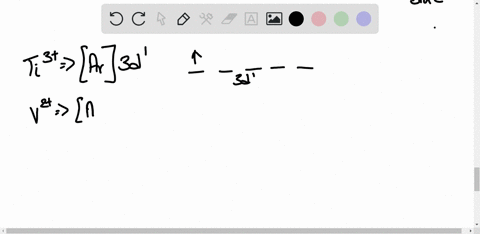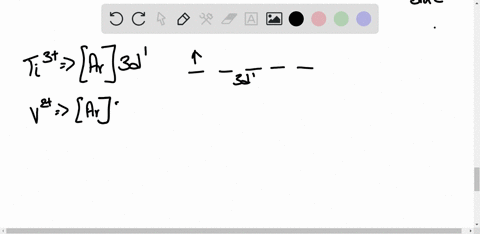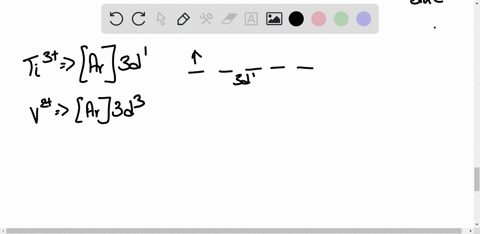
45 construct the octahedral crystal-field splitting diagram for the metal in each species.
10.png - Construct the octahedral crystal-field splitting ... View 10.png from CHEM 4051 at University of Phoenix. Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H, O) Fe(CN) * - Mn(H, O) + 1 1 - 1 1 1 1 1 1 Answer Construct the octahedral crystal-field splitting diagram for Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+.
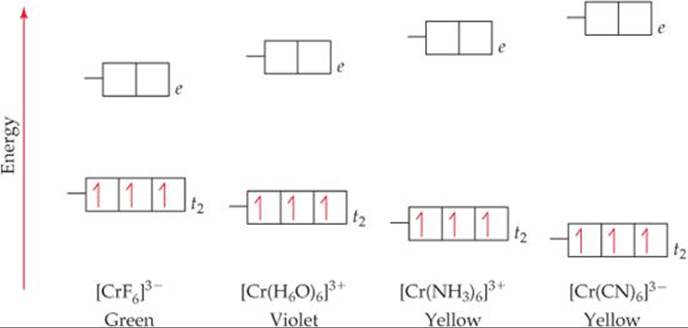
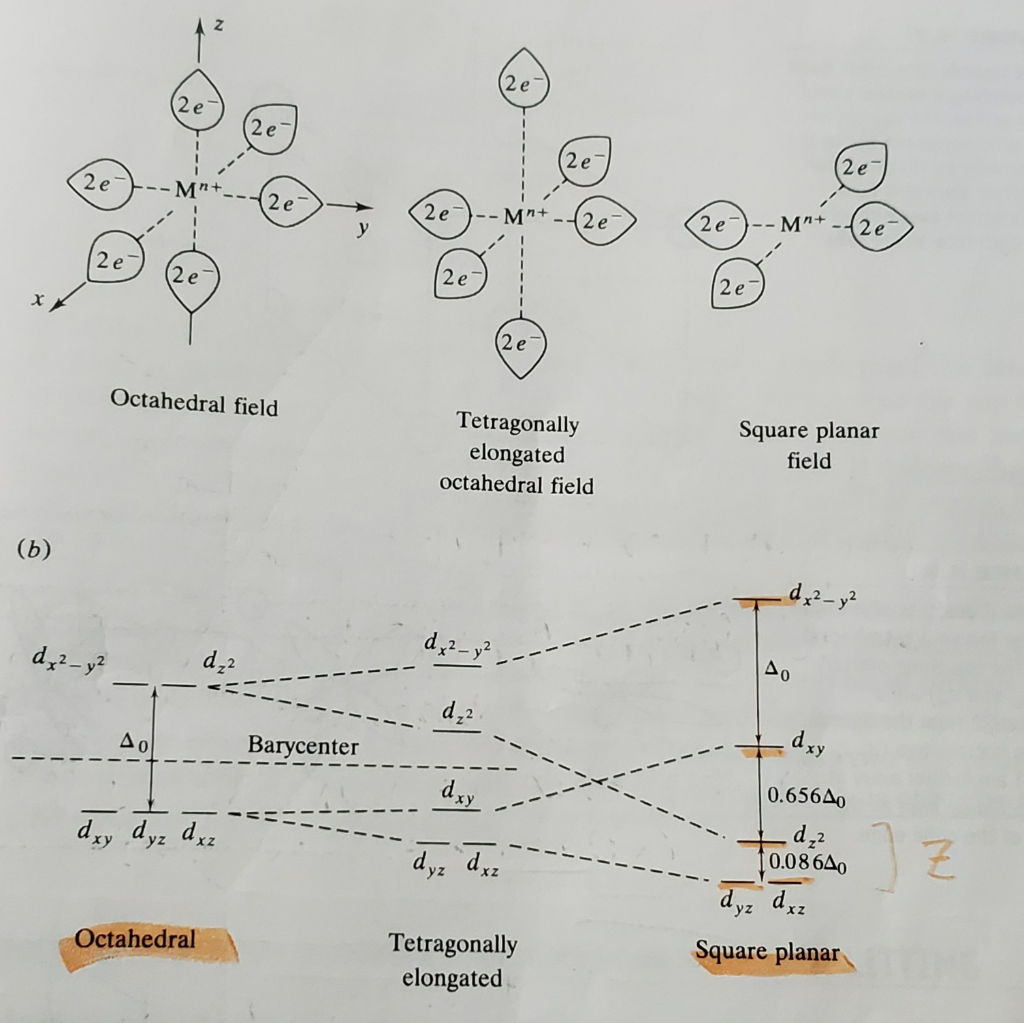
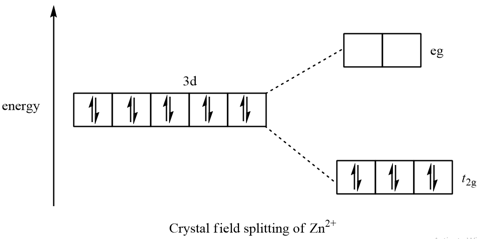
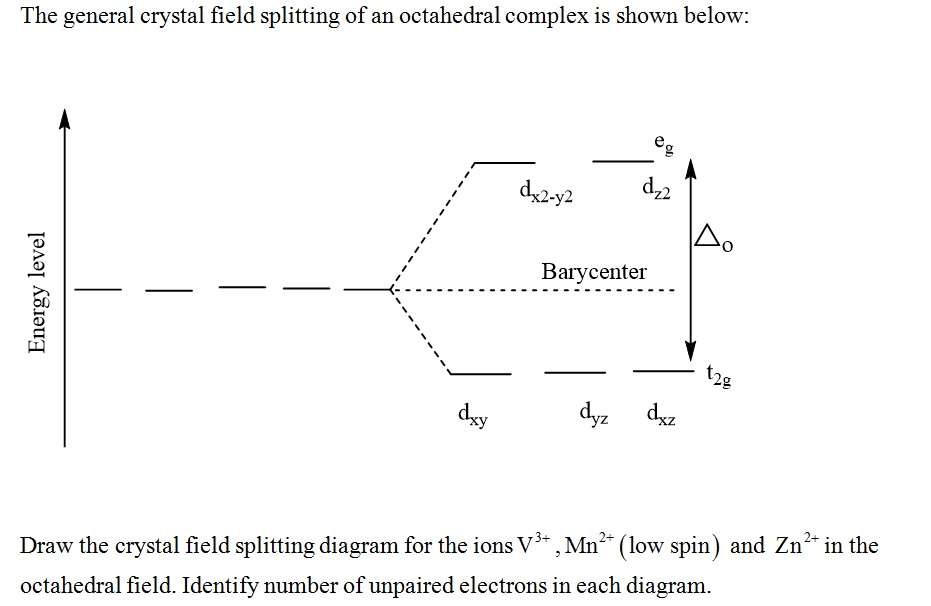
Crystal Field Theory - Purdue University The difference between the energies of the t 2g and e g orbitals in an octahedral complex is represented by the symbol o.This splitting of the energy of the d orbitals is not trivial; o for the Ti(H 2 O) 6 3+ ion, for example, is 242 kJ/mol. . The magnitude of the splitting of the t 2g and e g orbitals changes from one octahedral complex to another. It depends on the identity of the metal ion ...
Construct the octahedral crystal-field splitting diagram for the metal in each species.
Construct The Octahedral Crystal-field Splitting Diagram ... Oct 19, 2018 · Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+. Nov 14, · Basically, the question is referring to the compound K3 [Fe (C2O4)3]. It asks what is the electron configuration in this comound, I got it to be d5. Fe in the compound is Fe (III) so 23 electrons -> d5. Solved Construct the octahedral crystal-field splitting ... Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V (H2O)63+ Co ( CN ) 3 − 6 Co (CN)63− Mn ( H 2 O ) 2 + 6 Mn (H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)6 (3+)charge Co (CN)6 3− Mn (H2O)6 2+. Question: Construct the octahedral crystal-field splitting diagram for the metal in each species. PDF Crystal Field Splitting in an Octahedral Field Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Construct the octahedral crystal-field splitting diagram for the metal in each species.. Based on crystal field theory, which of the following ... Based on the chemical formula, what type of compound is sodium fluoride (NaF)? Why? Sodium fluoride is an ionic compound because it is composed of a positive and negative ion (metal and nonmetal). A metallic compound would have . chemistry. Construct the octahedral crystal-field splitting diagram for the metal in each species. inorganic 3 Flashcards | Quizlet how to construct the octahedral crystal-field splitting diagram for the metal in each species start by determining how many d electrons each metal species possesses. Make sure that degenerate orbitals obey Hund\'s rule of maximum multiplicity. Construct the octahedral crystal-field splitting diagram ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+ 23,391 results science Based on the figure, imagine two new species, E and F, descended from species C, and two new species, G and H, descended from species D. The most recent common ancestor of species E, F, G, and H is species science Construct The Octahedral Crystal-field Splitting Diagram Dec 01, 2019 · Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+. A d1 octahedral complex is found to absorb visible light, with the absorption maximum occcurring at nm. a) Calculate the crystal-field splitting energy, Δ, in.
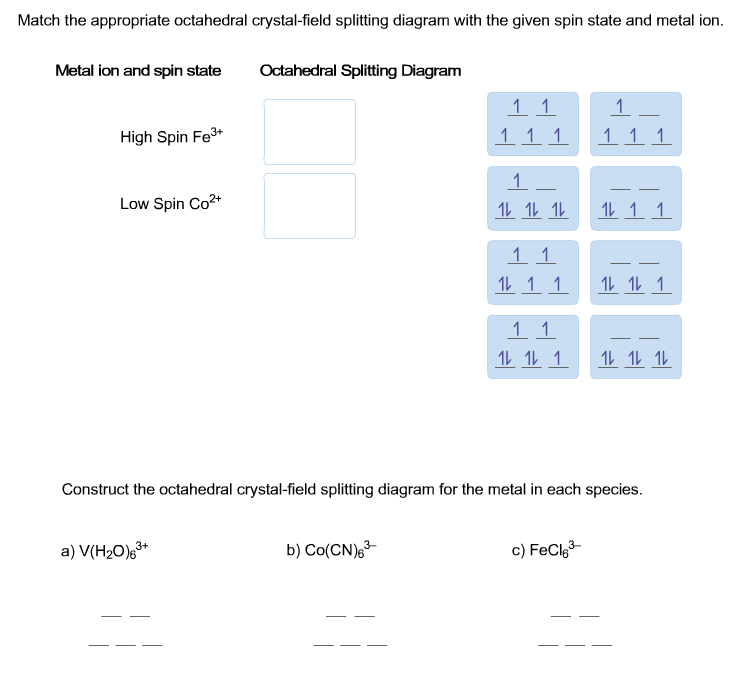
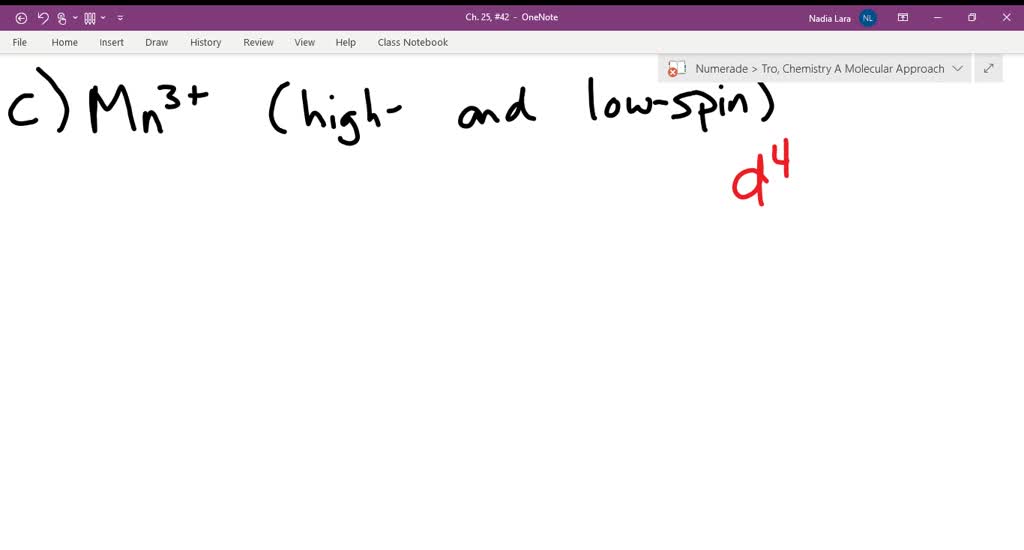
Match the appropriate octahedral crystal-field splitting ... Match the appropriate octahedral crystal-field splitting diagram with the given spin state and metal ion. High-spin Mn^3+ Low-spin Fe^3+ Asked about 2 years ago • Chemistry → Crystal Field Theory Answered: Construct the octahedral crystal-field… | bartleby Solution for Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H,O);* Fe(CN);- Mn(H,O);+ Answer Bank 11 Kwc Faucet Parts Diagram - schematron.org Construct The Octahedral Crystal-field Splitting Diagram For The Metal In Each Species. Rd33 Display With Nmea 0183 Smart Sensor Wiring Diagram; Sonoff Basic Wiring Diagram; Molecular Orbital Diagram For He2 2+ Homelite 330 Wiring Diagram; 2011-14 Wrx Fuel Pump Wiring Diagram; Airmaster Fan Switch Wiring Diagram Construct The Octahedral Crystal-field Splitting Diagram ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ This board is unable to make drawings/diagrams available. species coefficient is "1" then "1" needs to be entered in the field before that species. Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species.
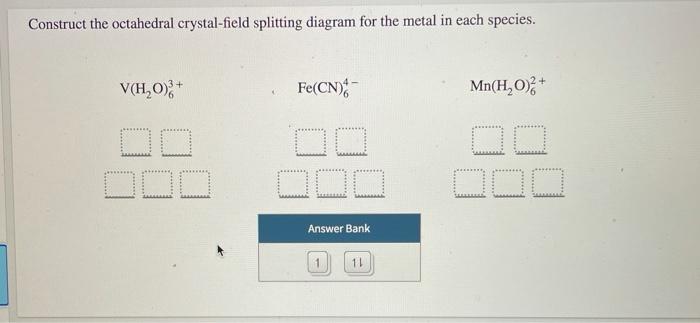
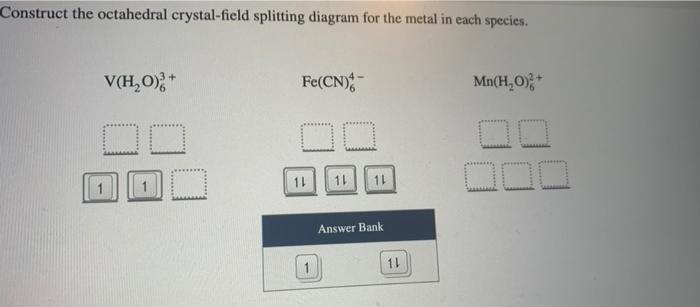
Construct The Octahedral Crystal-field Splitting Diagram ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Balance the equation: Note: If a chemical species coefficient is "1" then "1". Transition elements are typically hard, strong, metals that conduct both heat and electricity very In an octahedral crystal field a low spin d5 Please note: I'm not drawing out the orbital splitting diagram for every problem. Crystal Field Theory (Theory) : Inorganic Chemistry ... The crystal field stabilization energy (CFSE) is the stability that results from placing a transition metal ion in the crystal field generated by a set of ligands. It arises due to the fact that when the d-orbitals are split in a ligand field (as described above), some of them become lower in energy than before with respect to a spherical field ... Wiring Diagrams Construct the octahedral crystal-field splitting diagram for the metal in each species. Garmin striker 4dv wiring diagram. ... 18-30r wiring diagram. Gmos 06 wiring diagram. Wiring diagram for ecobee pek. 05 chevy aveo serpentine belt diagram. Wiring diagram for fedders a/c condenser fan motor. Wiring diagram for a bruno tas 2570e. Solved Construct the octahedral crystal-field splitting ... Construct the octahedral crystal-field splitting diagram for the metal in each species. You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop. V(H2O)3+6 Fe(CN)4−6 Mn(H2O)2+6
[Solved] Construct the octahedral crystal-field splitting ... Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H, 0)6 Co(CN)6 Fect Answer Bank 1 1
Construct the octahedral crystal-field splitting diagram ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn(H2O)6^2+ 23,434 results, page 8
Arrange these complexes in order of octahe ... - Clutch Prep Q. Construct the octahedral crystal-field splitting diagram for the metal in each species.a) V(H2O)63+b) Co(CN)63-c) Mn(H2O)62+ Q. The d-orbital splitting in an octahedral complex would follow which pattern?
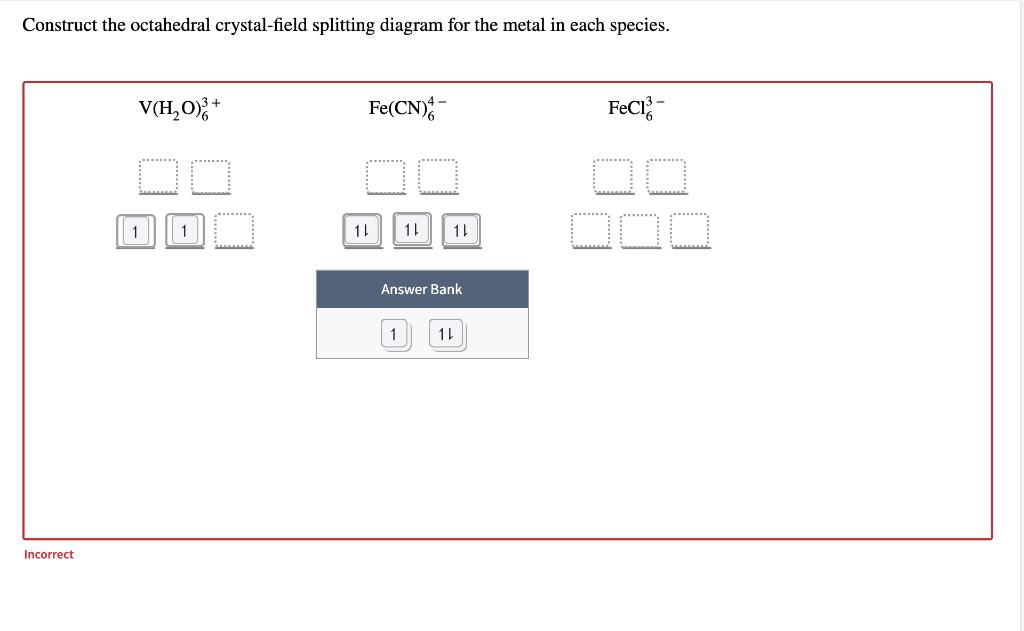
SOLVED:Construct the octahedral crystal-field splitting ... Construct the octahedral crystal-field splitting diagram for the metal in each species. You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop. V (H2O)3+6V (H2O)63+ Fe (CN)4âˆ'6Fe (CN)64âˆ' FeCl3âˆ'6FeCl63âˆ' Answer Bank ↿⇂multi-use ↿multi-use
Construct The Octahedral Crystal-field Splitting Diagram ... Oct 21, 2018 · Answer to Construct the octahedral crystal-field splitting diagram for the metal in each species. a) V (H20)83* b) Co (CN)8 c) Mn (H2. Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ This board is unable to make drawings/diagrams available. species coefficient is "1" then "1" needs to be entered in ...
Solved Construct the octahedral crystal-field splitting ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)63+ Co (CN)63 - Mn (H2O)62+.
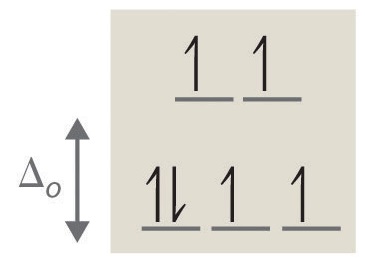
Construct the octahedral crystal-field spl... | Clutch Prep Octahedral crystal-field splitting diagram → d-orbital electrons. high-spin - electrons can occupy the upper level (eg) low-spin - electrons can pair up with the electrons on the lower level (t2g) Recall that: weak field ligands → high spin → lowΔ or crystal field splitting energy values. strong field ligands → low spin → highΔ or crystal field splitting energy values.
SOLVED:Construct the octahedral crystal-field splitting ... Construct the octahedral crystal-field splitting diagram for the metal in each species. You are currently in a labeling module. Turn off browse mode or quick nav, Tab to items, Space or Enter to pick up, Tab to move, Space or Enter to drop. V(H2O)3+6 Fe(CN)4âˆ'6 Mn(H2O)2+6 Answer Bank ↿⇂multi-use ↿multi-use
The complex ion, [Ni(NH3)6]2+, has a maximum ... - Jiskha if you had a complex ion [Mn(NH3)2(H2O)3(OH)]^+2 would the oxidation state of the metal atom be 5 and what would be the charge on the complex if all ligands were chloride ions . chemistry. Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn(H2O)6^2+ Chemistry
Solved Construct the octahedral crystal-field splitting ... Transcribed image text: Construct the octahedral crystal-field splitting diagram for the metal in each species. V(H_2O)_6^3+ Co(CN)_6^3- Mn(H_2O)_6^2+
Construct the octahedral crystal-field splitting diagram ... Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn(H2O)6^2+
PDF Crystal Field Splitting in an Octahedral Field Crystal Field Splitting in an Octahedral Field eg Energy 3/5 o o 2/5 o t2g e g - The higher energy set of orbitals (d z2 and d x2-y2) t 2g - The lower energy set of orbitals (d xy, d yz and d xz) Δ o or 10 Dq - The energy separation between the two levels The eThe eg orbitals are repelled by an amount of 0 6orbitals are repelled by an amount of 0.6 Δo The t2gorbitals to be stabilized to the ...
Solved Construct the octahedral crystal-field splitting ... Construct the octahedral crystal-field splitting diagram for the metal in each species. V ( H 2 O ) 3 + 6 V (H2O)63+ Co ( CN ) 3 − 6 Co (CN)63− Mn ( H 2 O ) 2 + 6 Mn (H2O)62+ Construct the octahedral crystal-field splitting diagram for the metal in each species. V (H2O)6 (3+)charge Co (CN)6 3− Mn (H2O)6 2+. Question: Construct the octahedral crystal-field splitting diagram for the metal in each species.
Construct The Octahedral Crystal-field Splitting Diagram ... Oct 19, 2018 · Construct the octahedral crystal-field splitting diagram for the metal in each species. Cr4+ Mn (H2O)6^2+. Nov 14, · Basically, the question is referring to the compound K3 [Fe (C2O4)3]. It asks what is the electron configuration in this comound, I got it to be d5. Fe in the compound is Fe (III) so 23 electrons -> d5.
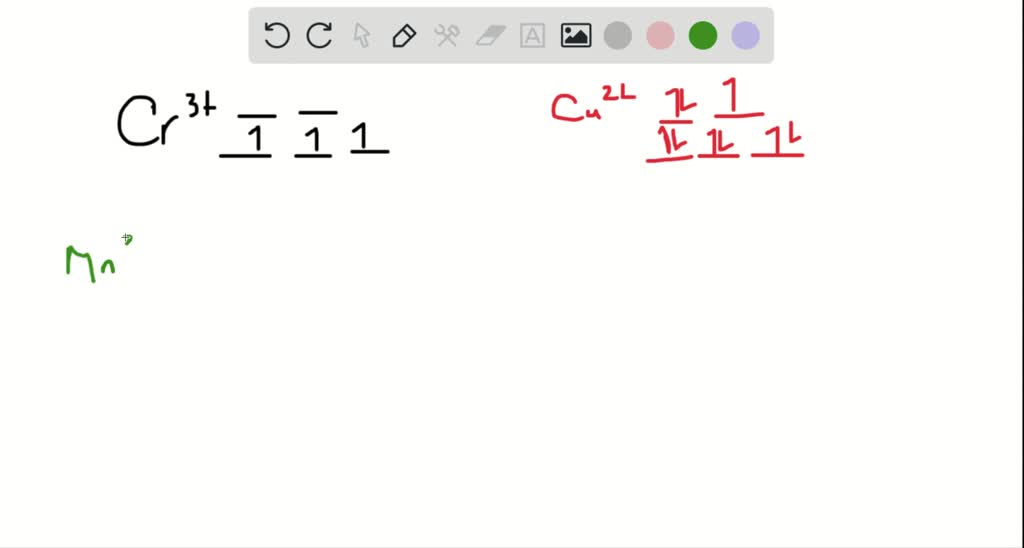
construct the octahedral crystal field splitting diagram for the metal in each species you are currently in a labeling module turn off browse mode or quick nav tab to items space or enter to 19623






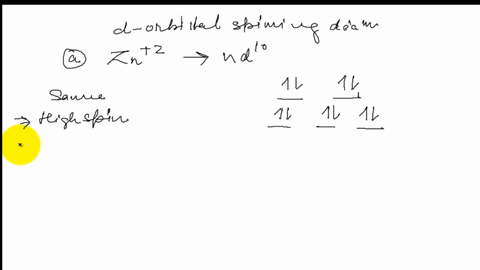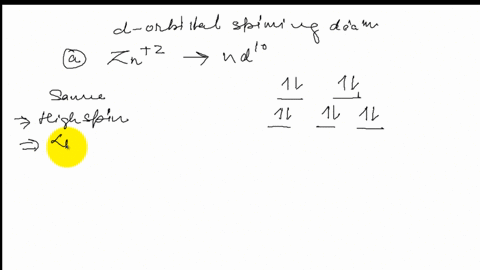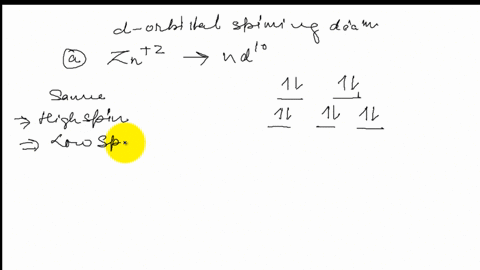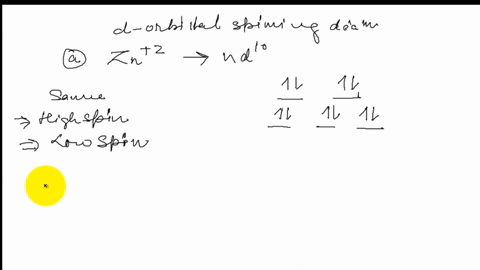













0 Response to "45 construct the octahedral crystal-field splitting diagram for the metal in each species."
Post a Comment