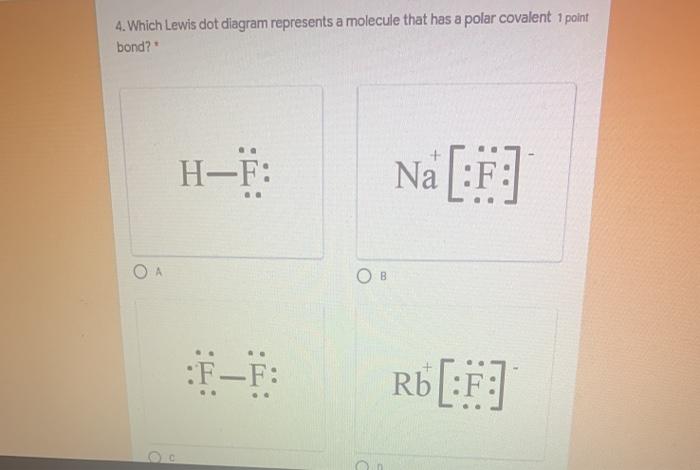
43 which electron dot diagram represents a polar molecule
Unit 4 Bonding Exam Name - The Leon M. Goldstein High ... 12.12.2017 · e) In the box below, draw the Lewis electron-dot structure for the compound formed from magnesium and chlorine. [ Include any charges or partial charges.] (1 pt.) 32) Explain, in terms of electronegativity, why an H-F bond is expected to be more polar than an H-I bond. (2 pts.) BONUS Questions – 1 pt. each 33) Given the reaction: H2 + Cl 2 2HCl Chemistry B Flashcards - Quizlet 2. an electron or particle similar to an electron emitted from the nucleus with negligible mass and a charge of -1 3. high energy ray of energy emitted from some radioactive atoms that is not affected by a magnetic field 4. neutral particle found in the nucleus; mass about equal to that of a proton 5. the central core of the atom containing most of the mass and made up of protons and …
Lewis Structure Questions and Answers | Study.com number of shared electron pairs b. number of unshared electron pairs c. shape of molecule d. ionic, polar covalent, or nonpolar covalent . View Answer. Write …

Which electron dot diagram represents a polar molecule
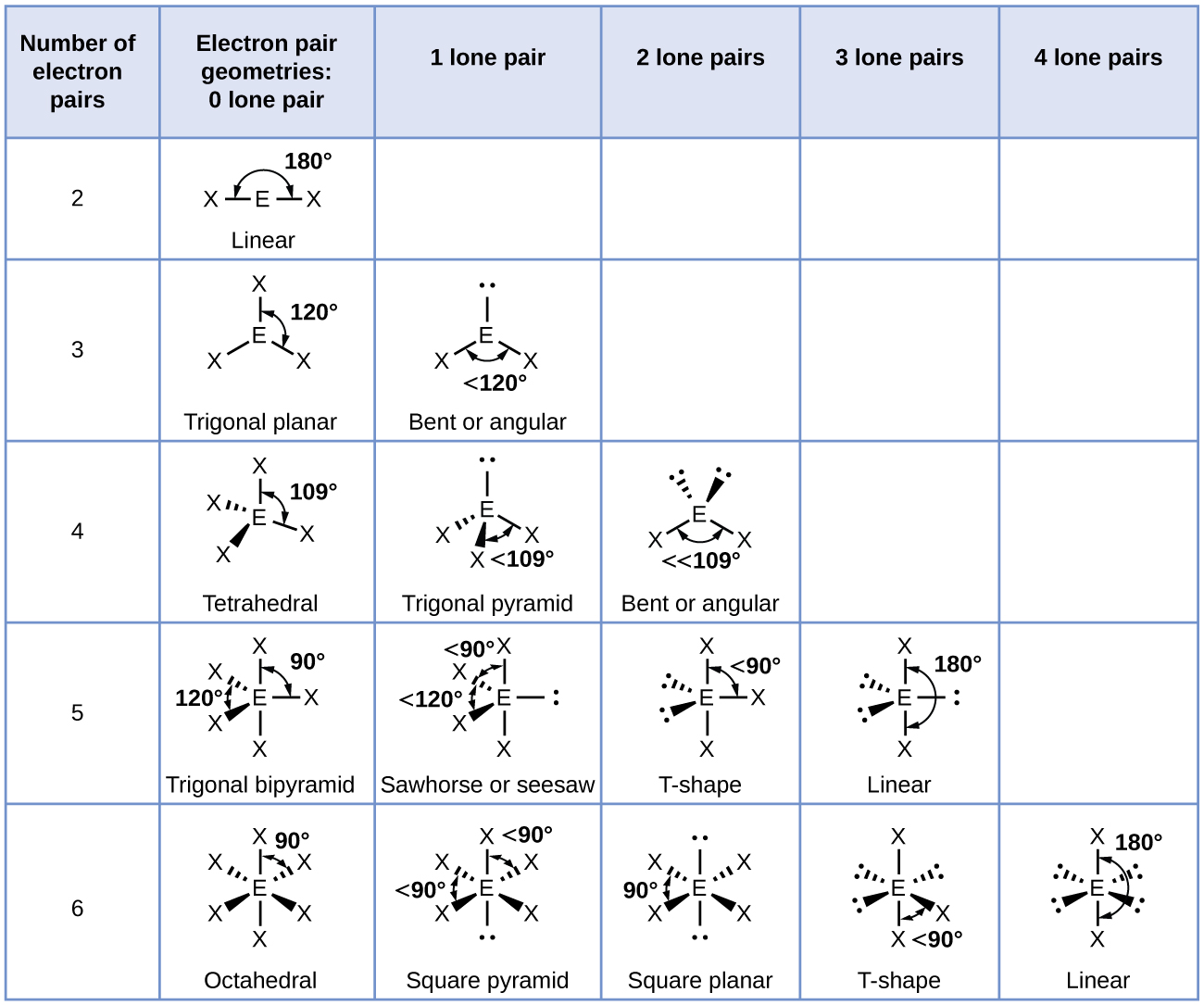
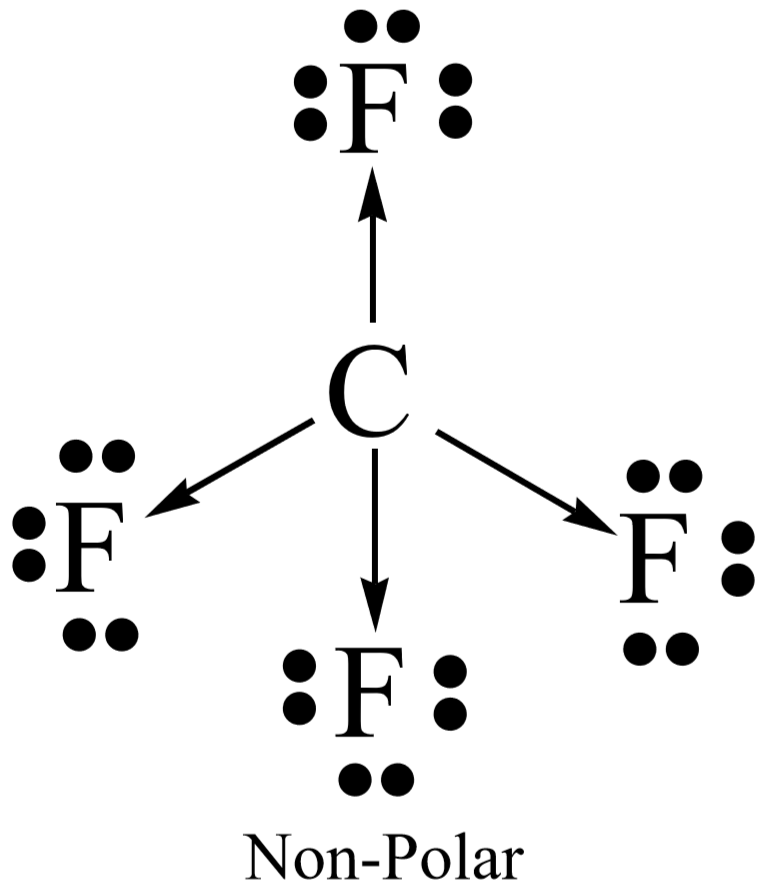
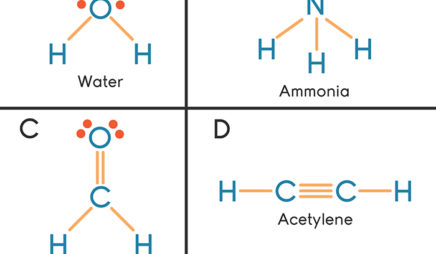
CO2 lewis structure, molecular geometry, bond angle, polar ... A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Let’s see how to draw a CO2 lewis dot structure with simple steps. Follow some steps for drawing the Lewis dot structure for CO2 1. Count total valence electron in CO2. As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. … Geometry of Molecules - Chemistry LibreTexts 21.08.2020 · A molecule is polar when the electrons are not distributed equally and the molecule has two poles. The more electronegative end of the molecule is the negative end and the less electronegative end is the positive end. A common example is HCl. Using the capital sigma + or - as a symbol to show the the positive end and the negative end we can draw the … Ethene (C2H4) lewis dot structure, molecular geometry ... X represents the bonded pairs of electrons to the central atom. N represents the lone pairs of electrons on the central atom; So, according to the C2H4 Lewis structure, carbon is the central atom that has 3 bonded pairs and 0 lone pairs of electrons. Hence, we got the AX 3 formula for C2H4. As per the VSEPR chart, if any molecule has an AX 3 formula then the molecular and …
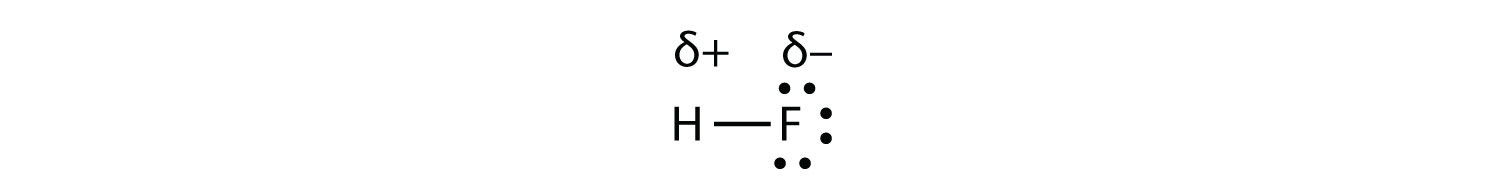
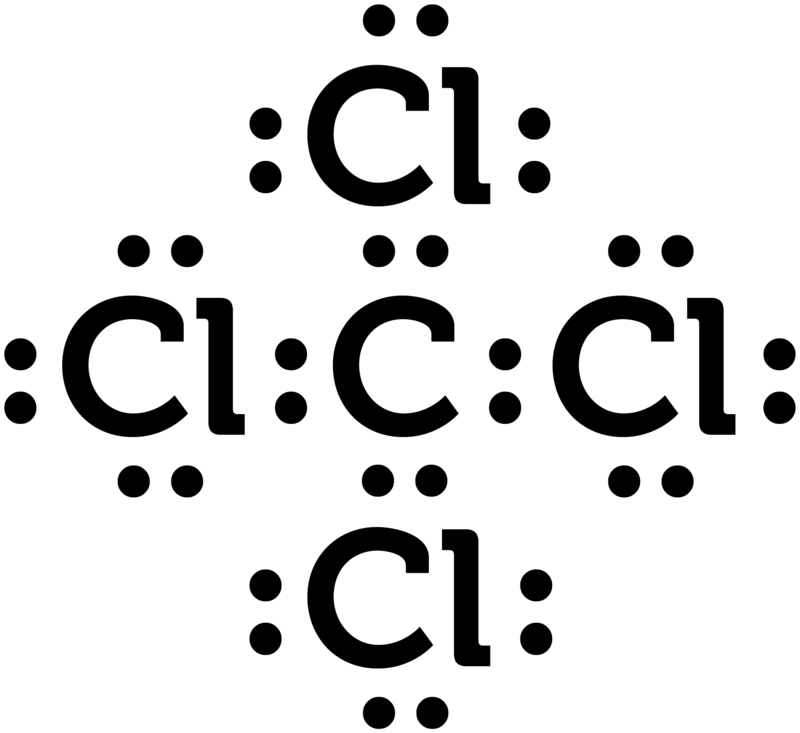
Which electron dot diagram represents a polar molecule. CS2 Lewis Structure, Hybridization ... - Techiescientist 09.03.2022 · Lewis Structure is one of the key terminologies to understand the chemical bonding of a molecule since it represents the molecular structure. It depends on the octet rule concept and is an extension of the electron dot diagram. Thus, to have a comprehensive idea about CS2 Lewis Structure, let us go through each step clearly and systematically. Step 1: The very first … HCOOH Lewis Structure, Molecular Geometry, Hybridization ... 10.03.2022 · The Lewis structures or electron dot structures are the two-dimensional diagrams, which represent the bonding electron pairs between atoms of the molecule and lone pairs of electrons on an atom if present. The bonding and nonbonding electrons are valence electrons, which are present in the outermost shell of an atom. As per the Lewis rule or octet rule, an … Covalent Bond: Types of Bonds, Examples, Formation - Embibe 17.01.2022 · Covalent bonding in molecular substances is represented by the Lewis electron dot diagram. For example, the Lewis diagram of two hydrogen atoms is: When two hydrogen atoms share electrons, the lewis diagram is as shown below: This depiction of molecules sharing electrons is shown by using a dash. This dash represents a covalent bond. The hydrogen … Lewis Structures: Single, Double & Triple Bonds - Video ... 21.11.2021 · Lewis dot structures, as you have learned, are a way to diagram an element and easily show its valence electrons. A Lewis dot structure is a diagram that shows the valence electrons in an element.
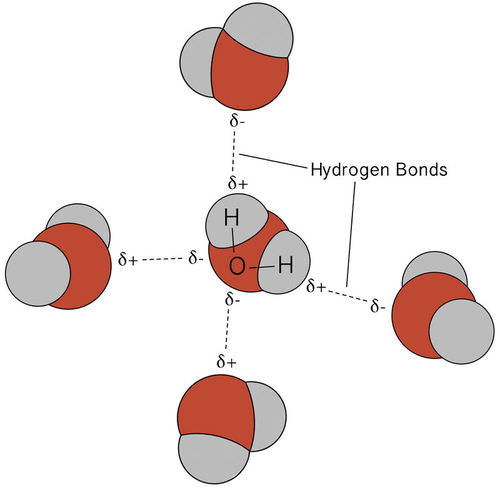
Ethene (C2H4) lewis dot structure, molecular geometry ... X represents the bonded pairs of electrons to the central atom. N represents the lone pairs of electrons on the central atom; So, according to the C2H4 Lewis structure, carbon is the central atom that has 3 bonded pairs and 0 lone pairs of electrons. Hence, we got the AX 3 formula for C2H4. As per the VSEPR chart, if any molecule has an AX 3 formula then the molecular and … Geometry of Molecules - Chemistry LibreTexts 21.08.2020 · A molecule is polar when the electrons are not distributed equally and the molecule has two poles. The more electronegative end of the molecule is the negative end and the less electronegative end is the positive end. A common example is HCl. Using the capital sigma + or - as a symbol to show the the positive end and the negative end we can draw the … CO2 lewis structure, molecular geometry, bond angle, polar ... A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Let’s see how to draw a CO2 lewis dot structure with simple steps. Follow some steps for drawing the Lewis dot structure for CO2 1. Count total valence electron in CO2. As we know, the lewis diagram is all about representing the valence electron of atoms within the molecule. …
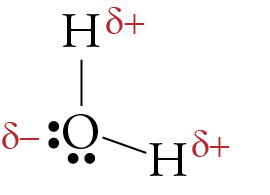







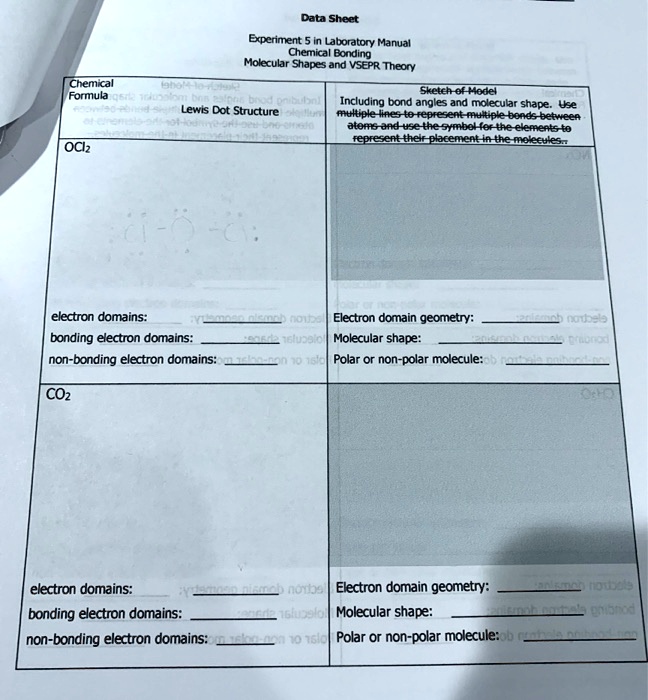




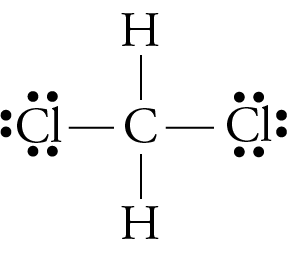





:max_bytes(150000):strip_icc()/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)


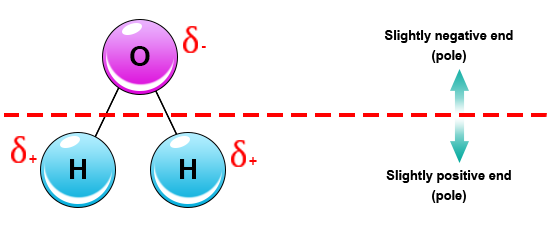




0 Response to "43 which electron dot diagram represents a polar molecule"
Post a Comment