43 phosphorus electron dot diagram
Lewis Dot Diagram Phosphorus - wiringall.com Lewis diagrams, also called electron-dot diagrams, are used to . Example: Draw the Lewis structure for phosphorus pentafluoride, PF5. The electron dot or Lewis dot structure of P4,which is the constituent molecule of white phosphorus,can be easily drawn keeping in mind the facts that: 1)It has.U.S. Department of Transportation (DOT ... PBr3 Lewis Structure, Molecular Geometry, Hybridization ... PBr3 Lewis Structure, Molecular Geometry, Hybridization and Polarity. PBr3 is a chemical formula for Phosphorus Tribromide. The molecule is made up of one Phosphorus atom and three Bromine atoms. It is a colourless liquid with a pungent odour. Like PF3 and PCl3, PBr3 also exhibits the properties of both Lewis Acid and Lewis Base.
What is the Lewis electron dot diagram for phosphorus? Jun 6, 2021 — So, to draw the Lewis Structure, begin by drawing the symbol for Phosphorus, the letter P. Next, Phosphorus has 5 valence electrons.
Phosphorus electron dot diagram
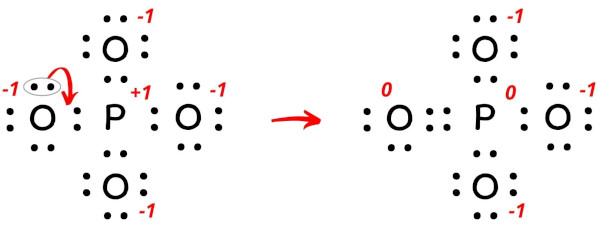
P2O5 (Phosphorus pentoxide) Lewis Structure Phosphorus is a group VA element in the periodic table and has five electrons in its last shell (valence shell). Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. valence electrons given by phosphorus atoms = 5 * 2 = 10 valence electrons given by oxygen atoms = 6 * 5 = 30 39 phosphorus electron dot diagram - Diagram Online Source Mar 19, 2022 · Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron. Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
Phosphorus electron dot diagram. Phosphorus trichloride | PCl3 - PubChem In a Hoechst continuous process, molten white phosphorus and gaseous chlorine react in previously produced phosphorus trichloride. The formation of phosphorus pentachloride is prevented by the presence of a small excess of phosphorus.The heat of reaction, ca. 10 times the heat of evaporation, keeps the system at its boiling point, and the phosphorus trichloride distills off. PF3 lewis structure, Molecular geometry, Polar or nonpolar ... Phosphorus trifluoride (PF3) lewis dot structure, molecular geometry, electron geometry, polar or nonpolar, bond angle. Phosphorus trifluoride appears as a colorless gas and it is highly toxic in nature similar to carbon monoxide or comparable to phosgene. It is odorless, nucleophile, and weak base in nature and has a chemical formula of PF3. PF5 Lewis structure, Molecular Geometry, Bond angle and ... The electron configuration of a Phosphorus atom in its ground state is 1s2 2s2 2p6 3s2 3p3, but when it is in an excited state, the electrons from 3s orbital get unpaired. There are five half-filled orbitals: one s orbital, three p orbitals, and one d orbital. All these five orbitals accommodate one valence electron of the Fluorine atoms. PCl5 Lewis Structure, Molecular Geometry, Hybridization ... Drawing the Lewis structure of PCl5. Step 1: Count the number of valence electrons in a PCl5 molecule. We can refer to the periodic table for this. We come to understand that PCl5 is made up of Phosphorous and Chlorine. Phosphorus, having atomic number 15, has an electron composition of 2, 8, 5. Therefore, it has 5 electrons in its outermost shell.
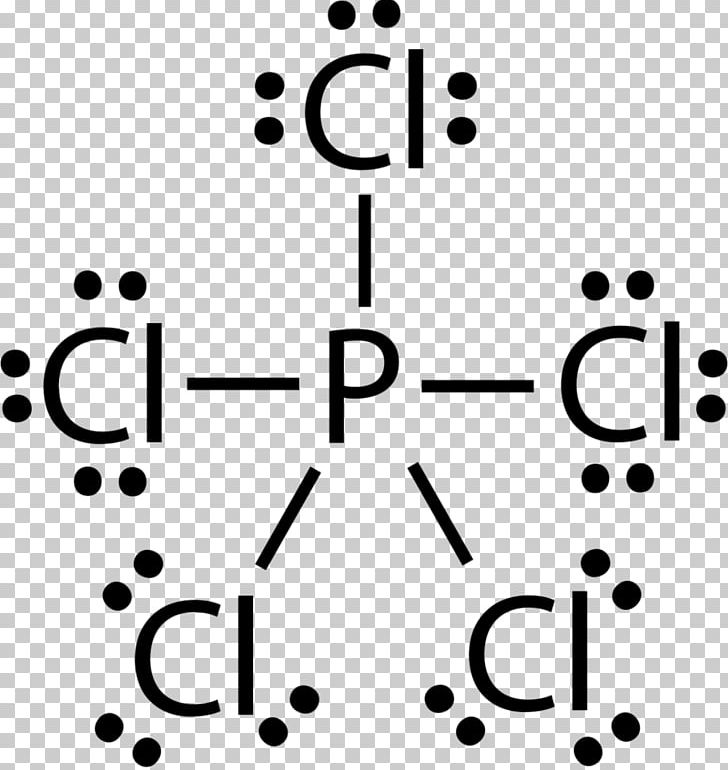
How to draw PCl3 Lewis Structure? - Science Education and ... The phosphorus atom in the molecule gets only 8 electrons around its molecular structure. This central phosphorus atom is octet stable. But it has one lone pair. Phosphorus is a brownish solid in nature. Phosphorus catch fire in the exposure to air. But phosphorus is used in matchboxes and firecrackers. Phosphorus Dot diagram - Summarized by Plex.page | Content ... Feb 04, 2022 · In the PCl5 Lewis model, there are a total of 40 valence electrons. On the Periodic table, Phosphorous is in Period 3 when you draw the Lewis structure for PCl5: Remember that Phosphorous is in Period 3 on the Periodic table when you lay it out. How many dots would go around potassium in an electron dot diagram? Phosphorus Draw the Lewis dot structure for ... - Study.com Lewis Structure: The Lewis structure of a chemical element is a schematic representation of the valence electrons it has on the atom. The dots are used to represent electrons which are present in ... PPTX AIM: How to write Lewis Dot Structures (Electron Dot ... Lewis Dot Structure (Electron Dot Structure) A Lewis dot structure is a quick and easy diagram that shows the valence electrons in an element.In a Lewis structure, the nucleus of the element is represented by its symbol. The valence electrons are represented by dots placed around the symbol in pairs.
How many dots are drawn in the Lewis dot diagram for ... Jun 6, 2020 — Hereof, what is the Lewis dot structure for phosphorus? There are a total of 40 valence electrons in the PCl5 Lewis structure. 6.1 Lewis Electron Dot Diagrams | Introductory Chemistry A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... PBr3 Lewis Structure - How to Draw the Lewis Structure for ... A step-by-step explanation of how to draw the PBr3 Lewis Dot Structure (Phosphorus Tribromide).For the PBr3 structure use the periodic table to find the tota... What is the Lewis dot structure for PCl3? - handlebar ... We can clearly see from the lewis diagram that in PCl3, phosphorus is forming three sigma bonds with 3 chlorine atoms. With that 2 lone pairs are present on the phosphorus atom. This concept very well explains the hybridization of PCl3 which is sp3. How is the phosphorus trichloride ( PCl3 ) made?
Phosphorus Bohr Model - How to draw Bohr diagram for ... Electron dot diagram of a Phosphorus atom Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Phosphorus, we got to know, it has 5 valence electrons. So, just represent the 5 valence electrons around the Phosphorus atom as a dot. The electron configuration of Phosphorus
Phosphorus Mononitride Lewis Structure - Novocom.top Phosphorus Mononitride Lewis Structure, lewis dot phosphorus diagram compounds, diagram dot phosphorus mo diagrams lewis untpikapps electron argon cations fewer ions, charge formal valence atoms shells lewis structure phosphate expanded why bond lone don pairs stack, mononitride phosphorus
9.1 Lewis Electron Dot Diagrams What is the Lewis electron dot diagram for each element? phosphorus; argon. Answer. For atoms with partially filled d or f subshells, these electrons are ...
PF3 Lewis Structure, Molecular Geometry, and Hybridization The Lewis structure is drawn using eight dots of valence electrons around the symbols of the atom with lines showing bond formation. PF3 is a tetra-atomic molecule where phosphorus donates three valence electrons, and three fluorine atoms accept one electron each to undergo a bond formation and reach a stable condition.
Lewis Dot Diagram Phosphorus - schematron.org Sep 23, 2018 · Since there are 4 electron pairs around phosphorus, the geometry is based upon a tetrahedron, but since one of these electron pairs is a stereochemically active non-bonding pair, the. Lewis diagrams, also called electron-dot diagrams, are used to represent paired and unpaired valence (outer shell) electrons in an atom.
what is the lewis dot structure for pbr3 - shapovmusic.com What is the molecular geometry molecular structure of pbr3? The answer is A. trigonal pyramidal. The central atom for the compound is P. Looking at the central atom, it will have 3 bonds involving the 3 Br… How many valence electrons are in the electron dot structure of pbr3? In the Lewis structure for PBr 3 there are a total of 26 valence ...
How to draw PBr3 Lewis Structure? - Science Education and ... The phosphorus atom in the molecule gets only 8 electrons around its molecular structure. This central phosphorus atom is octet stable. But it has one lone pair. Phosphorus is a brownish solid in nature. Phosphorus catch fire in the exposure to air. But phosphorus is used in matchboxes and firecrackers.
PF5 Lewis Structure - Learnool PF5 Lewis Structure. PF 5 (phosphorus pentafluoride) has one phosphorus atom and five fluorine atoms. In the lewis structure of PF 5, there are five single bonds around the phosphorus atom, with five fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs.
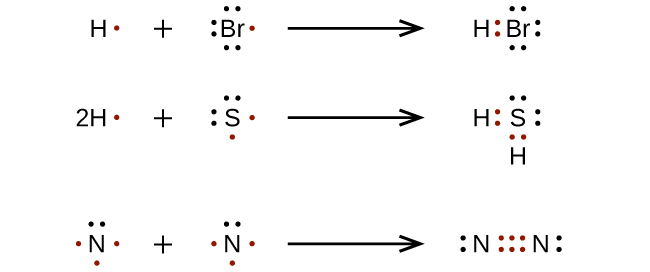
Electron Dot (Lewis) Diagrams - Mr. Sault's Classroom 1. Draw an “electron dot” diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal.
PCl3 (Phosphorus Trichloride) Lewis Structure Phosphorus is a group VA element in the periodic table and has five electrons in its last shell (valence shell). Chlorine is a group VIIA element in the periodic table and contains seven electrons in its last shell. valence electrons given by phosphorus atom = 5 * 1 = 5 valence electrons given by oxygen atoms = 7 * 3 = 21
Lewis Dot Diagram Magnesium And Phosphorus - Novocom.top lewis structure phosphorus atom dot electron draw electrons valence system dots satisfy wps facilitates represent uses socratic . Advertisiment. phosphorus quiz diagrams highlight weebly differences . ionic magnesium calcium lewis phosphide nitride sulfide . magnesium phosphate formula chloride .
Electron Configuration for Phosphorus (P) - UMD In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the ...
Lewis Dot Diagram For Phosphorus - schematron.org Dec 26, 2018 · More information about the DOT ID/UN number and the guide number can be found at the Emergency Response Guidebook. Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron.
Electron Dot Diagrams | Chemistry for Non-Majors Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
39 phosphorus electron dot diagram - Diagram Online Source Mar 19, 2022 · Nov 22, · Best Answer: The Lewis Electron Dot Diagram for Phosphorous is P with 5 dots around it. There are 5 valence electrons, so this is how many dots are around. Each dot represents one electron.
P2O5 (Phosphorus pentoxide) Lewis Structure Phosphorus is a group VA element in the periodic table and has five electrons in its last shell (valence shell). Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. valence electrons given by phosphorus atoms = 5 * 2 = 10 valence electrons given by oxygen atoms = 6 * 5 = 30




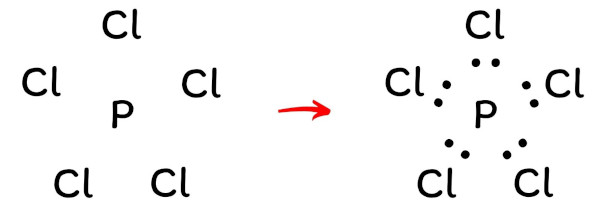
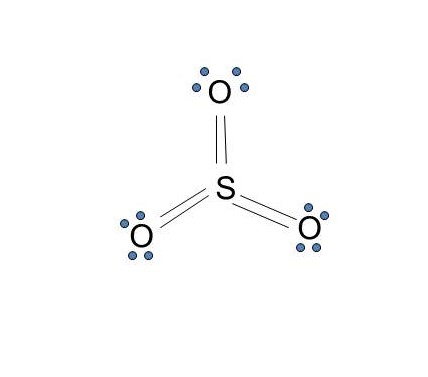

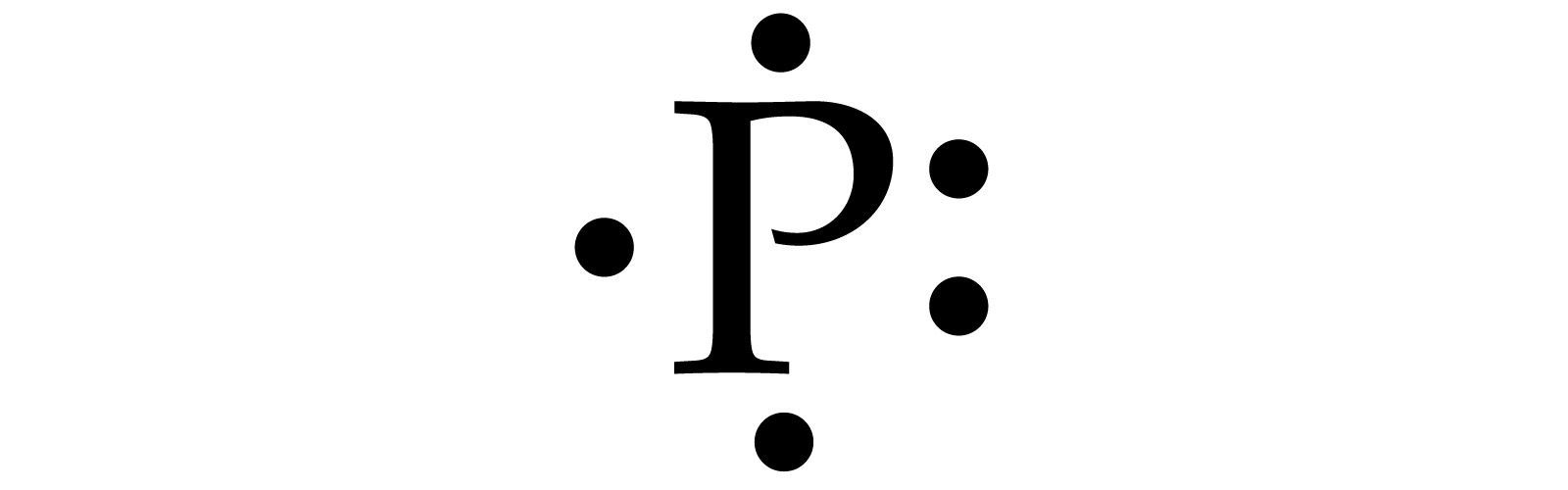







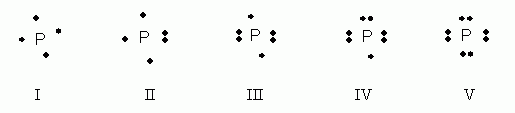


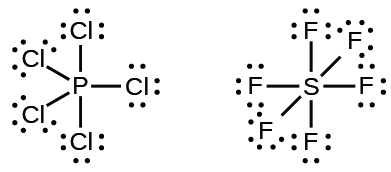

/Lewis-dot-58f78f405f9b581d5938e617.jpg)














0 Response to "43 phosphorus electron dot diagram"
Post a Comment