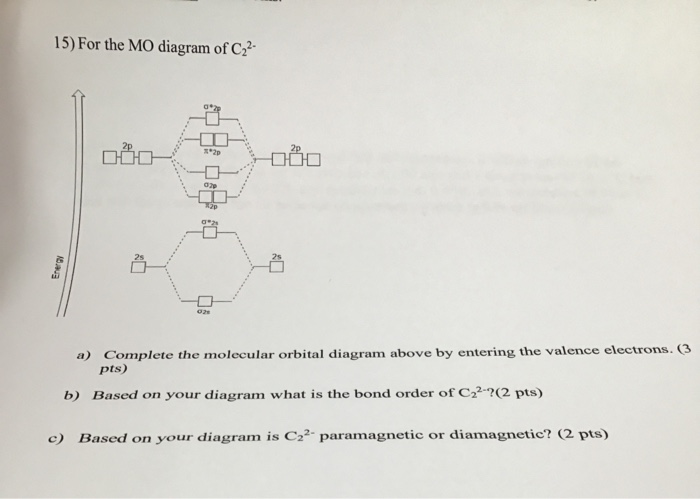
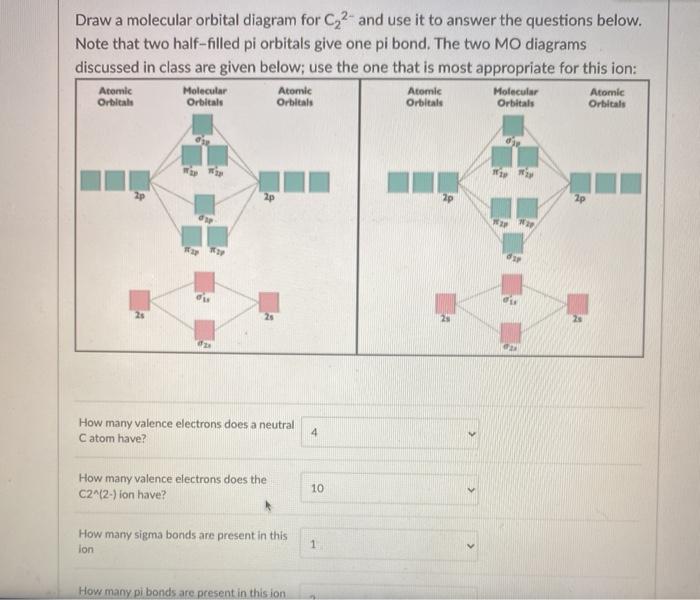
43 c2 2- molecular orbital diagram
Energy level diagram for Molecular orbitals - Chemical Bonding and... The molecule is diamagnetic. The double bond in C2 consist of both Pi bonds because the four electrons are present in the two pi molecular orbitals. Diagram for O2+ is wrong because 2p atomic orbital of 2nd O atom will have only 3 e-. Reply. Mrs Shilpi Nagpal says. Introduction to Inorganic Chemistry/Molecular Orbital Theory... Valence bond (VB) theory gave us a qualitative picture of chemical bonding, which was useful for predicting the shapes of molecules, bond strengths, etc. It fails to describe some bonding situations accurately because it ignores the wave nature of the electrons.
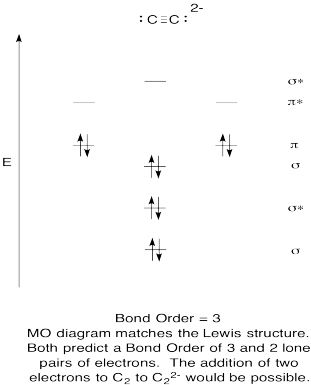
Molecular Orbital (MO) Diagram for C2(2-) - YouTube When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so...

C2 2- molecular orbital diagram
PDF A qualitFaetIIive molecular orbital diagram SfAoLrC 'sferrocene (D5d) A qualitative molecular orbital diagram for ferrocene (D5d) FeII SALC's Fe. • The attachment of additional groups or ligands destroys the D5d/D5h symmetry of ferrocene thus significantly altering the MO diagram. [Solved] The molecular orbital diagram for the carbide... | Course Hero Q: Use the molecular orbital diagram shown to determine which of the following is paramagnetic. None of the ions are para. Q: Can you answer the Twelve Chemical Reactions part 1-12 and Eleven Elegant Elements part 1-11 and the Ten Magnificent Mol. PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
C2 2- molecular orbital diagram. Molecular orbital diagram for BF3 - Chemistry Stack Exchange I'm trying to build a molecular orbital diagram for BF3 and I'm running into problems with irreducible representations on the F side. 2p (along the bond axis) for F has an irreducible representation of E' and A' 2p (z) for F Has an irreducible representation of E'' and A''2 2p (the remaining one) has an... Paramagnetic vs Diamagnetic | Forum To answer the question you have to draw the molecular orbital diagram or electron configuration for OF+ and NO+ (not the lewis For a molecule to be paramagnetic, there has to be at least one unpaired electron in a molecular orbital. Molecular Orbital Theory (MOT), Chemistry Study... | eMedicalPrep For example, homonuclear diatomic molecules of second row elements like Li2, Be2, B2 , C2, N2 , the σ 2pz MOs is higher in energy than π 2px and π 2py MOs. No. 9 Molecular Orbital Diagram for CO. Analysis done by Bond Order. If value of bond order is positive, it indicates a stable molecule and if... PDF Microsoft PowerPoint - Polyatomic Molecular Orbital Theory... Molecular Orbital Theory - linear XH2 molecules. • BeH2 (D∞h). The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180o to 90o.
Molecular Orbital Theory | Boundless Chemistry Molecular orbital diagrams are diagrams of MO energy levels, shown as short horizontal lines in the center. Atomic orbitals (AO) energy levels are shown for comparison. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels. PDF Procedure for Constructing Molecular Orbital Diagrams Based on... Last time you learned how to construct molecule orbital diagrams for simple molecules based on the symmetry of the atomic orbitals. We could use the symmetry-based method to construct molecular orbital diagrams for larger molecules as well, but this can get complicated for larger structures. By molecular orbital theory, bond order of c2 is 2, while... - Quora The orbital diagram for a diatomic molecule is. To find the bond order, add the 15 electrons in the molecular orbitals (the blue-colored energy levels in Molecular Orbital Theory shows that there are two sets of paired electrons in a degenerate pi bonding set of orbitals. This gives a bond order of 2... 8.4 Molecular Orbital Theory - Chemistry 2e | OpenStax Molecular Orbital Theory. considers bonds as localized between one pair of atoms. considers electrons delocalized throughout the entire molecule. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8.34).
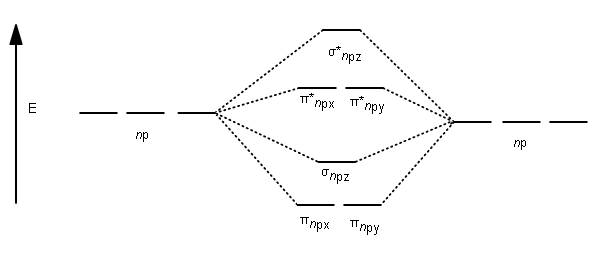
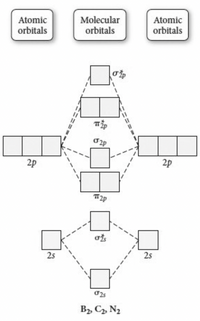
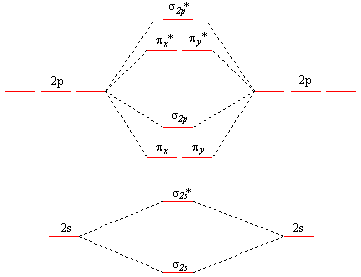
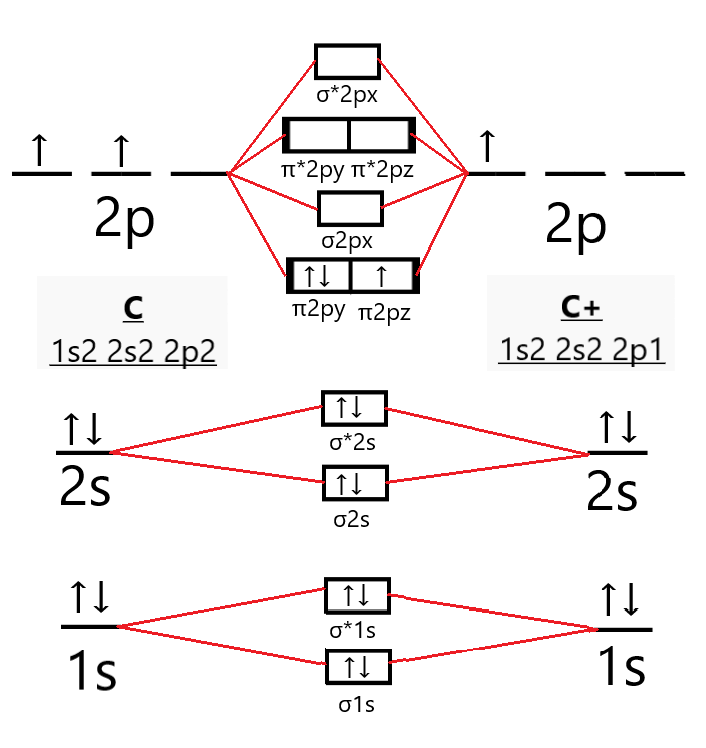
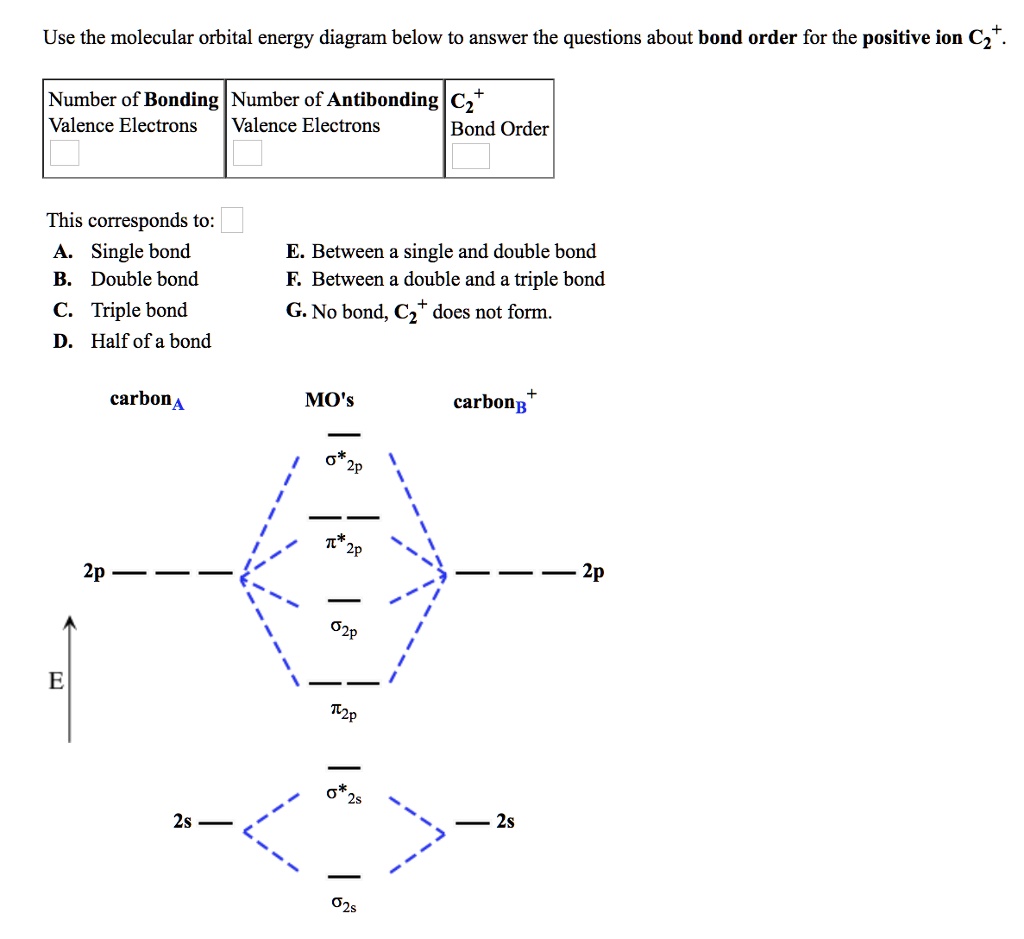
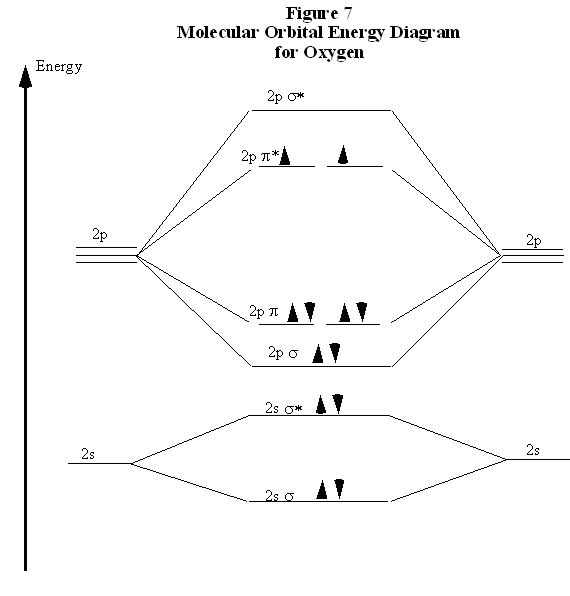
What is the molecular orbital diagram for C_2^-? | Socratic The lowest energy unoccupied molecular orbital is 2p_(sigma), so that is where the extra electron will be added. The problem provides you with the MO diagram for the #"C"_2# molecule, so all you really have to do here is add an electron to that diagram. PDF Molecular Orbitals in | 9-2 Molecular Orbital Energy Level Diagrams In a bonding molecular orbital, the electron density is high between the two atoms, where it stabilizes the arrangement by exerting a strong attraction for both Figure 9-2 Molecular orbital (MO) diagram for the combination of the 1s atomic orbitals on two identical atoms (at the left) to form two MOs. MO Diagrams | Molecular Orbital Diagram Maker A bare molecular orbital diagram is presented and you must drag the correct orbitals and labels onto the diagram. The diagram is then completed by filling the energy levels with the correct number of electrons. The following molecules are currently available: Molecules of the First Row Molecular Orbital Theory The molecular orbital diagram for an O2 molecule would therefore ignore the 1s electrons on both oxygen atoms and concentrate on the interactions between the 2s and 2p Experiments have shown that O2 and F2 are best described by the model in the figure above, but B2, C2, and N2 are best...
Molecular Orbital Diagrams simplified | by Megan A. Lim | Medium Molecular Orbital Diagrams simplified. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding the difference between two major theories: Valence Bond Theory and Molecular Orbital Theory.
Polyatomic Species | Molecular Orbital Theory | Chemogenesis Polyatomic species like methane, CH4, can be described in terms of molecular orbital theory, however, the diagrams can be very difficult to visualise. However, structures built up from hybrid atomic orbitals are much easier comprehend.
PDF Microsoft Word - Handin8s2017ans.docx 1. Sketch the qualitative molecular orbital diagram for XeF2. The molecule is linear and symmetric. Assume the valence 5s-orbitals of Xe are sufficiently lower in energy than the valence 5p-orbitals that the valence 5s-orbital of Xe and the 2s-orbitals of the F-atoms form an inner core 2 C1 S 0.00000.
Molecular orbital diagram - Wikipedia A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular.
PDF Molecular Orbital Diagrams A second molecular orbital is also created, which we simplistically show as a subtraction of the two atomic 1s orbitals [σ* = (1sa - 1sb)]. There would be four electrons to fill into our molecular orbital diagram and that would force us to fill in the bonding sigma MO and the anti-bonding sigma-star MO.
Molecular Orbitals of The Allyl Cation, Allyl Radical, and Allyl Anion But what happens to the molecular orbital diagram if we add a third contributing p-orbital? Or if we have an adjacent double bond, contributing 2 further p-orbitals, giving us 4 conjugated p You've heard of methyl, ethyl, propyl, and butyl; our name for the three carbon unit H2C=CH-CH2 is "allyl".
Tutorial on Chemical Bonding, Part 8 of 10 (Molecular orbitals) This second orbital is therefore called an antibonding orbital. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H2+.
Asked for: molecular orbital energy-level diagram, valence electron... Although the molecular orbital theory is computationally demanding, the principles on which it is based are similar to those we used to determine electron configurations for atoms. The two available electrons (one from each H atom) in this diagram fill the bonding σ1s molecular orbital.
PDF Molecular | 90" (porbitals). This dilemma has been resolved by orbital . . 2 molecular orbital calculations elec MOLECULAR ORBITAL and valence bond calculations of the w-electron energies of unsaturated molecules custom-arily start with models in which appropriate atomic orbitals a r e assigned to each nucleus to provide a framework for -notions...
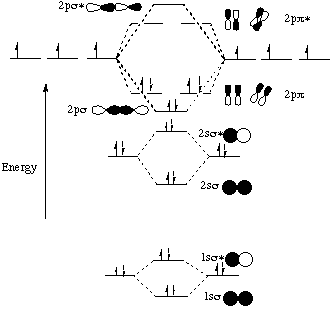
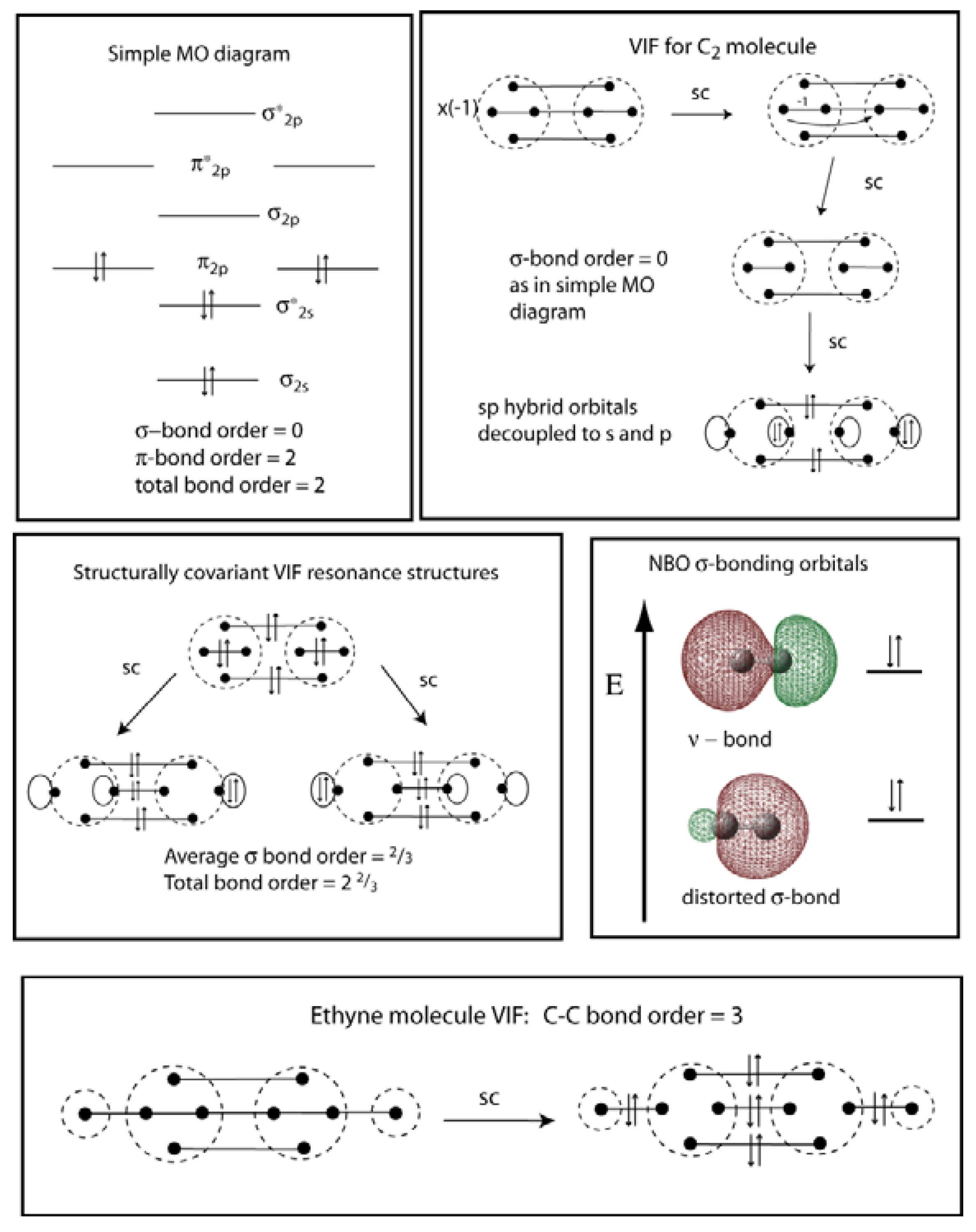
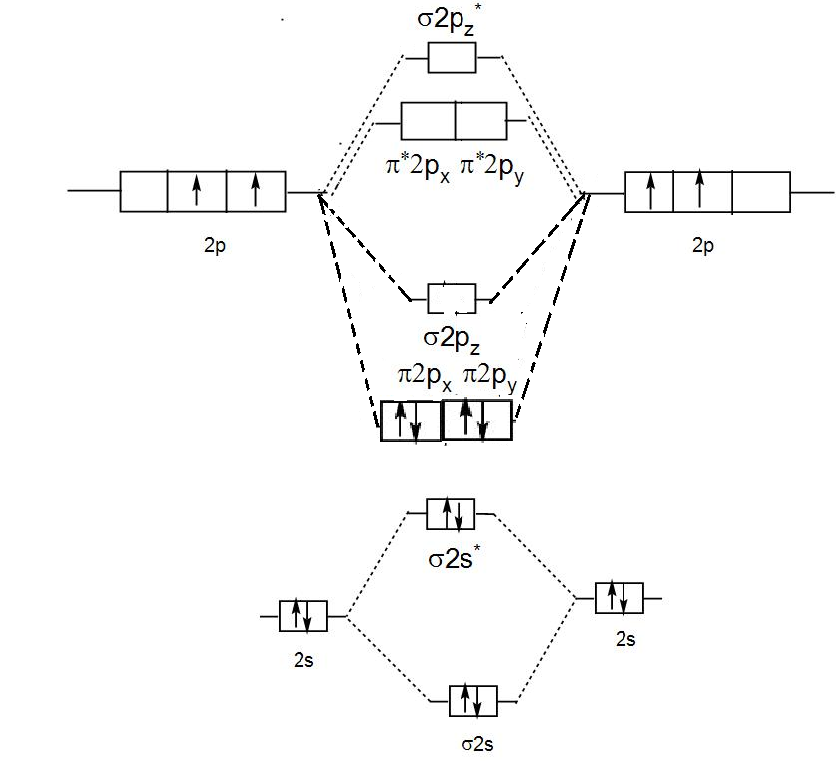
Molecular Orbital diagram of NO(nitric oxide) molecule Molecular orbital : A molecule in which all the electrons are paired, is called diamagnetic. | Online Chemistry tutorial IIT, CBSE Chemistry, ICSE Chemistry, engineering and medical chemistry source : isite.lps.org. Molecular orbital diagram of C2 molecule : Number of electrons in C2 molecule = 12.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired... Molecular Orbital Energy Diagrams. The relative energy levels of atomic and molecular orbitals are typically shown in a molecular orbital diagram (Figure 8). For a diatomic molecule, the atomic orbitals of one atom are shown on the left, and those of the other atom are shown on the right.
PDF Microsoft PowerPoint - An introduction to Molecular Orbital Theory.ppt... • Bonding - Review VSEPR and Hybridisation - Linear combination of molecular orbitals (LCAO), bonding / antibonding - Labelling of • It is a waste of both the lecturers and students time if the tutorial to ends up being a lecture covering questions. 5. An introduction to Molecular Orbital Theory.
[Solved] The molecular orbital diagram for the carbide... | Course Hero Q: Use the molecular orbital diagram shown to determine which of the following is paramagnetic. None of the ions are para. Q: Can you answer the Twelve Chemical Reactions part 1-12 and Eleven Elegant Elements part 1-11 and the Ten Magnificent Mol.
PDF A qualitFaetIIive molecular orbital diagram SfAoLrC 'sferrocene (D5d) A qualitative molecular orbital diagram for ferrocene (D5d) FeII SALC's Fe. • The attachment of additional groups or ligands destroys the D5d/D5h symmetry of ferrocene thus significantly altering the MO diagram.






![Molecular orbital diagram of [Zr(C 2 O 4 ) 4 ] 4 ...](https://www.researchgate.net/profile/Jason-Holland-6/publication/261604290/figure/fig3/AS:296702067134465@1447750693241/Molecular-orbital-diagram-of-ZrC-2-O-4-4-4-dodecahedral-D-2d-symmetry-Note_Q640.jpg)


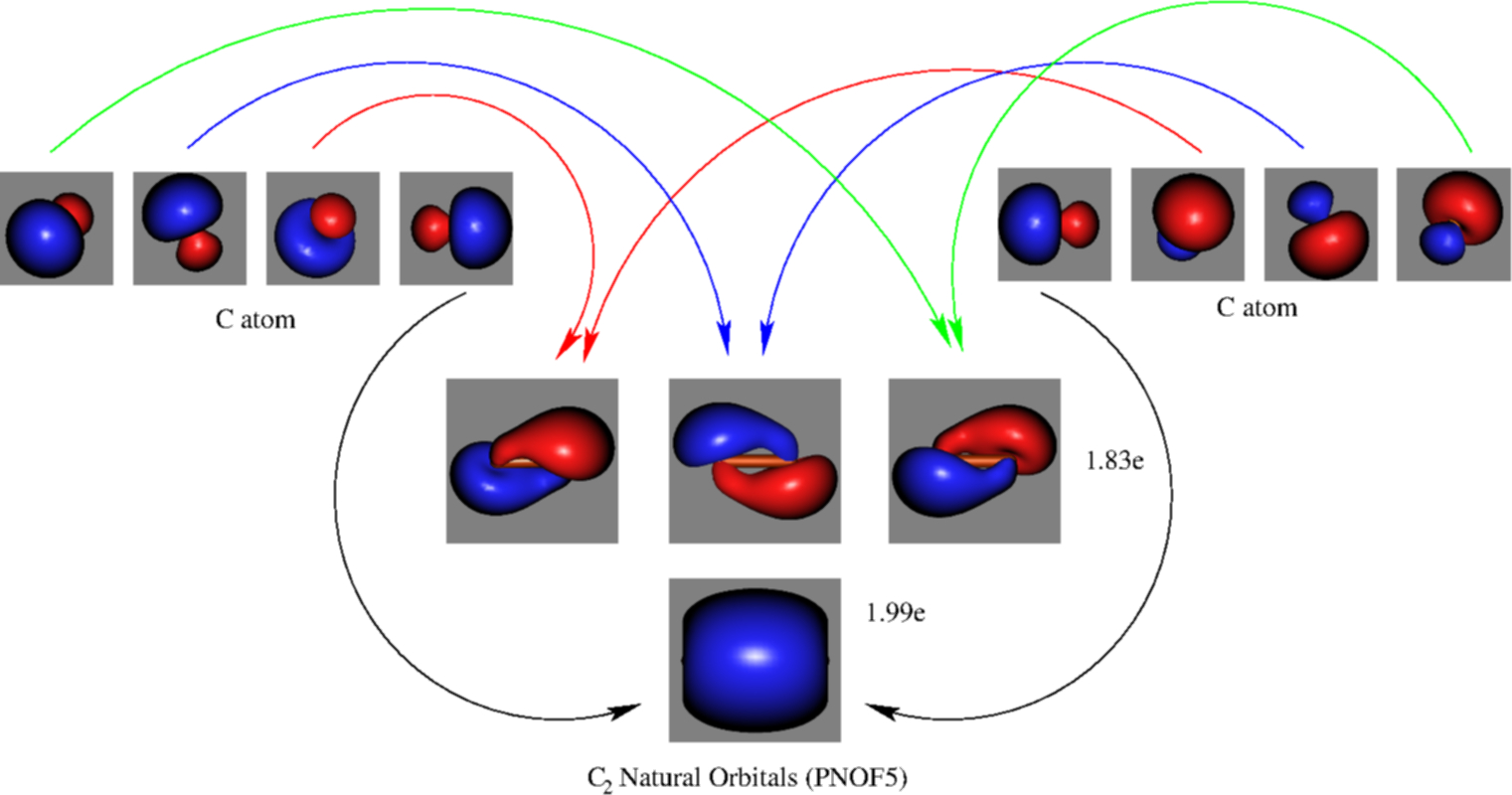


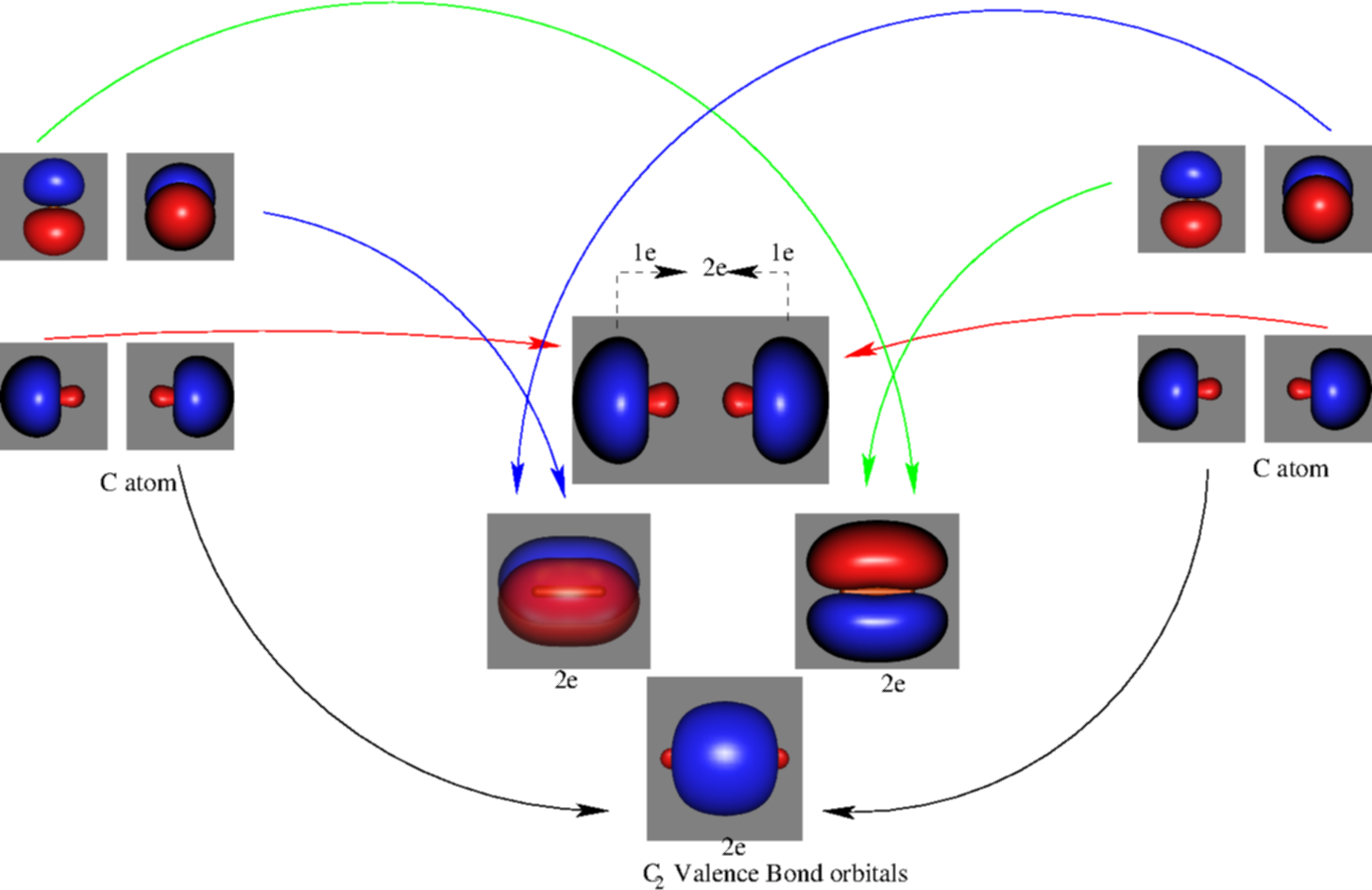






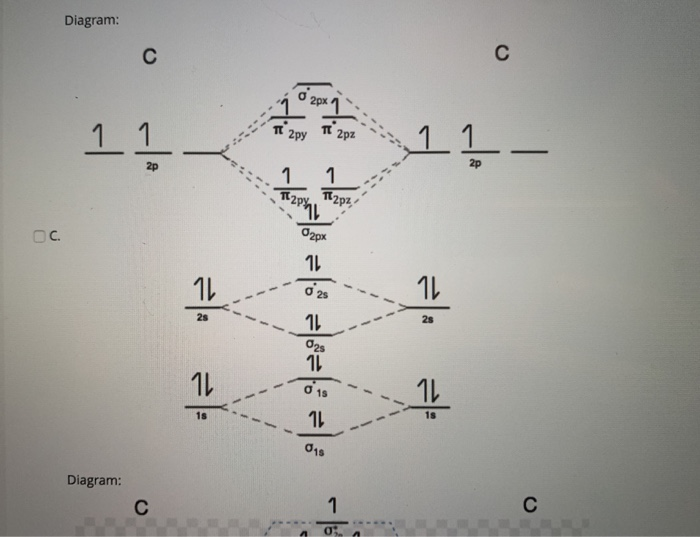
![Orbital descriptions of C2\documentclass[12pt]{minimal ...](https://www.researchgate.net/publication/337282871/figure/fig2/AS:958973922525208@1605648608866/Orbital-descriptions-of-C2documentclass12ptminimal-usepackageamsmath.png)

0 Response to "43 c2 2- molecular orbital diagram"
Post a Comment