42 lewis diagram for ch4
How to determine the Lewis structure for CH4 - Quora Answer (1 of 2): I would be lazy and look it up on the internet. But seriously, you have an electron pair between the C and each of the H's in the Lewis diagram a ala Why is that the correct diagram, you ask? First, each Hydrogen has only one electron to donate or share, and remember that Hydro... Functional Groups In Organic Chemistry 06.10.2010 · Functional groups are specific groupings of atoms within molecules that have their own characteristic properties, regardless of the other atoms present in a molecule. Common examples are alcohols, amines, carboxylic acids, ketones, and ethers. In a typical sophomore organic chemistry course, there’s about 14 functional groups that are key, with another group …
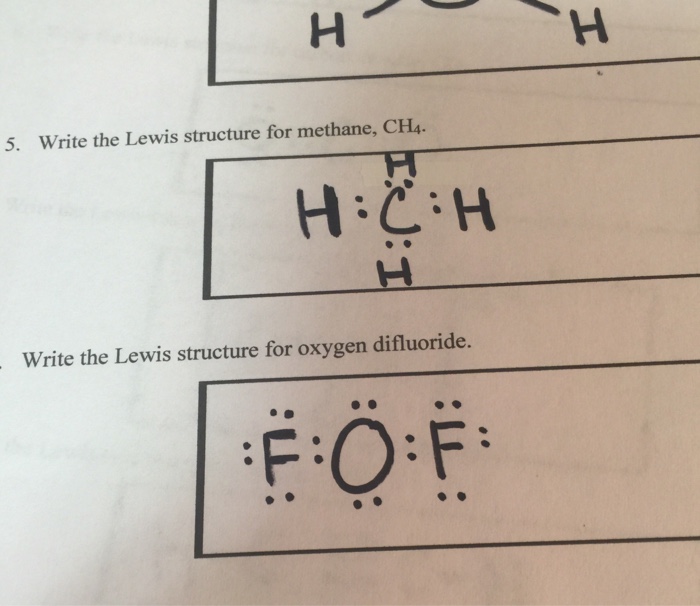
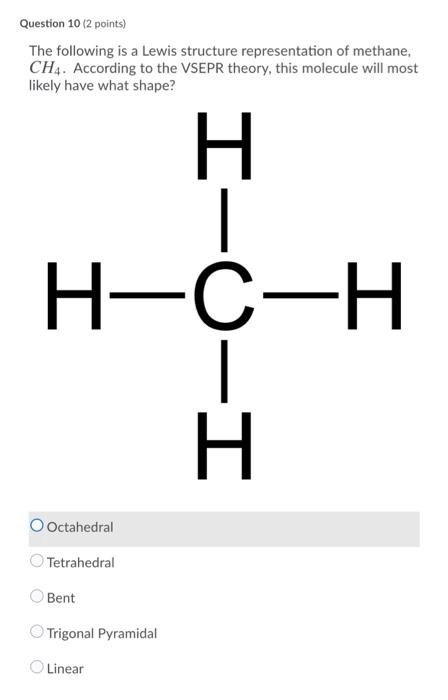
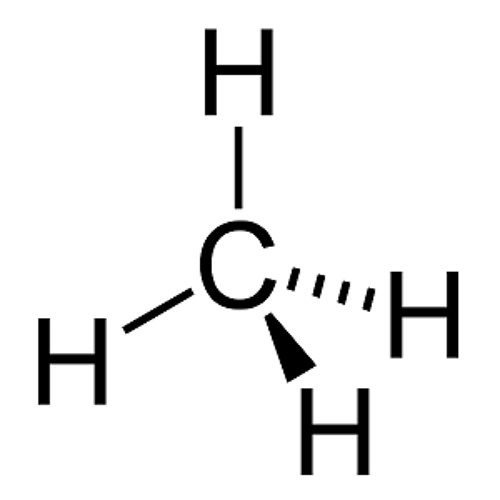
Solved Please note that "geometry" refers to the ... - Chegg The Lewis diagram for CH4 is: H-C-H The electron-pair geometry around the C atom in CH4 is There are lone pair (s) around the central atom, so the geometry of CH4 is Previous question Next question
Lewis diagram for ch4
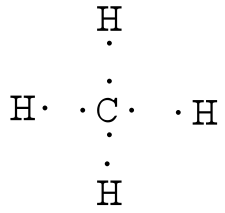
quizlet.com › 85211460 › chemistry-final-reviewChemistry Final Review Flashcards | Quizlet The diagram below shows gas with an initial pressure of 3060 mm Hg in a cylinder at a constant temperature. The gas expands inside the Cylinder and pushes the piston up. What is the final pressure of the gas after the expansion? Lewis Structure for CH4 (Methane) - UMD Drawing the Lewis Structure for CH 4 For CH 4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds. It's one of the easier Lewis structures to draw. Ch4 Electron Dot Diagram The electrons are arranged in four pairs representing four covalent bonds. The Lewis dot structure for CH 4 shows the number of valence electrons around each atom. Each dot represents a valence electron. The number of valence electrons . Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper.
Lewis diagram for ch4. CH4 Lewis Structure - How to Draw the Dot Structure for ... How to Draw the Lewis Dot Structure for CH4: MethaneA step-by-step explanation of how to draw the CH4 Lewis Dot Structure (Methane).For the CH4 structure use... topblogtenz.com › cyanide-cn-lewis-structureCN- lewis structure, molecular orbital diagram, and, bond order In this article, we will study the Cyanide (CN-) lewis structure, molecular orbital diagram(MO), its bond order, formal charges, and hybridization. Cyanide can be a colorless gas in the form of hydrogen cyanide, sodium cyanide, potassium cyanide, etc. MakeTheBrainHappy: The Lewis Dot Structure for CH4 The Lewis Dot Structure for CH4 is shown above. These kinds of structures can also be shown by representing each of the bonds with two dots. Each atom in the bond has a full valence, with carbon having access to eight electrons and each hydrogen having access to two (this is why hydrogen only needs two).The covalent bonds between the C and the H are similar to the ones formed between two Hs ... Ch4 Lewis Diagram - ch4 molecular geometry shape and bond ... Ch4 Lewis Diagram - 9 images - ch 4 carbon and diversity, ppt covalent bonding powerpoint presentation id 5648526,
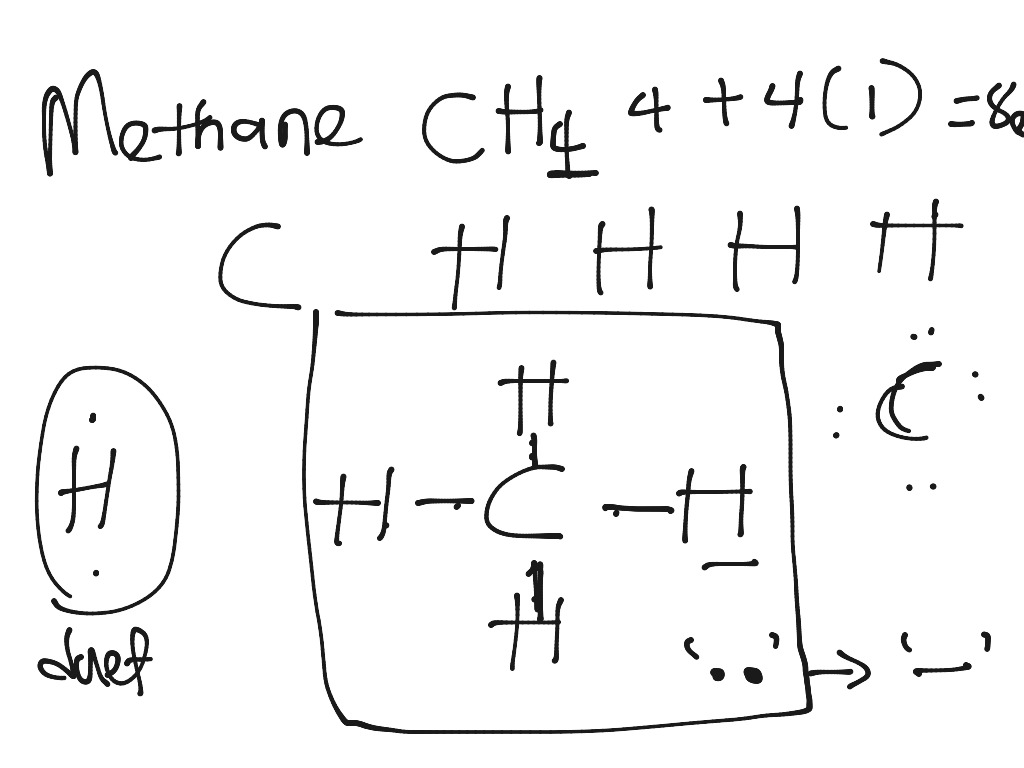
CH3COOH lewis structure, molecular geometry, polarity ... Also, by looking at the lewis diagram of acetic acid, its structure doesn’t seem to appear symmetrical, which means, it has unequal or unsymmetrical sharing of valence electrons. This unequal distribution of charge generates a net dipole moment which makes the CH3COOH molecule polar in nature. Acetic acid has a net dipole moment of 1.74 D which is close to … Lewis Dot Structure for CH4 | Chemical Bonding | Electron ... Dr. B. explains how to draw the Lewis dot structure for CH 4 (methane). The CH 4 Lewis Structure is one of the most frequently tested Lewis Structures.. Note that hydrogen atoms always go on the outside of a Lewis dot structure. This is because they can share a maximum of two electrons. Dot Diagram For Ch4 - Wiring Diagrams Lewis dot structure of CH 4. Alternatively a dot method can be used to draw the lewis structure. Calculate the total valence electrons in the molecule. C-4 H-1x4=4. Total=8. Put carbon in center and arrange hydrogen atoms on the wiringall.come electrons between carbon and hydrogen atoms. Dot Diagram For Ch4 - Wiring Diagram Pictures Lewis Dot Structure for CH4 #2 Find the number of "octet" electrons for the molecule. C: 8 octet electrons x 1 atom = 8 octet electrons H: 2 octet electrons x 4 .May 03, · A step-by-step explanation of how to draw the Lewis Structure Oxygen Gas (Dioxygen). For the O2 Lewis structure, calculate the total number of valence electrons for the ...
Lewis Dot Diagram Ch4 For CH 4 you have a total of 8 total valence electrons.. Drawing the Lewis structure for CH 4 (named methane) requires only single diagramweb.net's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. Stream CH4 in Lewis structure by ®© | Listen online for ... Stream CH4 in Lewis structure by ®© on desktop and mobile. Play over 265 million tracks for free on SoundCloud. quizlet.com › 498595002 › phase-diagrams-flash-cardsPhase Diagrams Flashcards | Quizlet Open the phase diagram for CO2 given in the introduction again. Use the phase diagram for CO2 in the interactive activity and determine which of the following statements are correct. CO2 is a gas under normal conditions of temperature and pressure. All three phases of CO2 exist simultaneously at the triple point. How Many Valence Electrons Are In Ch4 (Methane) Have ... The lewis structure of CH4 is drawn to fulfill the need of valence electron by every the atoms. Lewis structure of CH4. The lewis structure of carbon and hydrogen atom says- to form a solitary CH4 molecule, a complete of eight valence electrons take part in the common bonding to fulfill the require of eight an ext valence electrons.
CH4 Lewis Structure, Hybridization, Molecular Geometry ... CH4 Lewis Structure Lewis structure is the pictorial representation of the arrangement of valence shell electrons in the molecule, which helps us understand the atoms' bond formations. The electrons that participate in bond formation are called the bonding pair of electrons, while those that don't are known as nonbonding pairs of electrons.
topblogtenz.com › cl2-lewis-structure-molecularCl2 lewis structure, Molecular shape, Polar or Non-Polar, Dot ... Lewis dot structure for Cl2 (Chlorine gas) By looking at the above Cl2 lewis structure, we see both chlorine atoms completed their octet comfortably as both of them have 8 electrons around them. And no need to make any covalent bond in this lewis diagram because we got our stable lewis dot structure for Cl2.
wellcometreeoflife.org › co2-lewis-structureCO2 Lewis Structure (2021 UPDATED) All You Need To Know Dec 22, 2021 · In a Molecular Orbital Diagram, the 2s orbital of oxygen is nonbonding because of the high energy difference between carbon and oxygen atoms. Based on the rules of the Lewis Structure, all 16 electrons are filled upon bond formation, but the nonbonding orbitals remain vacant, as in the case of CO2.
C2H4 lewis structure, molecular geometry, bond angle ... C2H4 Lewis’s structure is very helpful to find Its molecular geometry because the lewis diagram helps us to determine how many bond pairs and lone pairs a molecule contains. Let’s see how to find this. Follow three steps to find C2H4 molecular geometry . 1. Find the Number of lone pairs present on the central atom of the C2H4 Lewis structure. The lone pair is also called the …
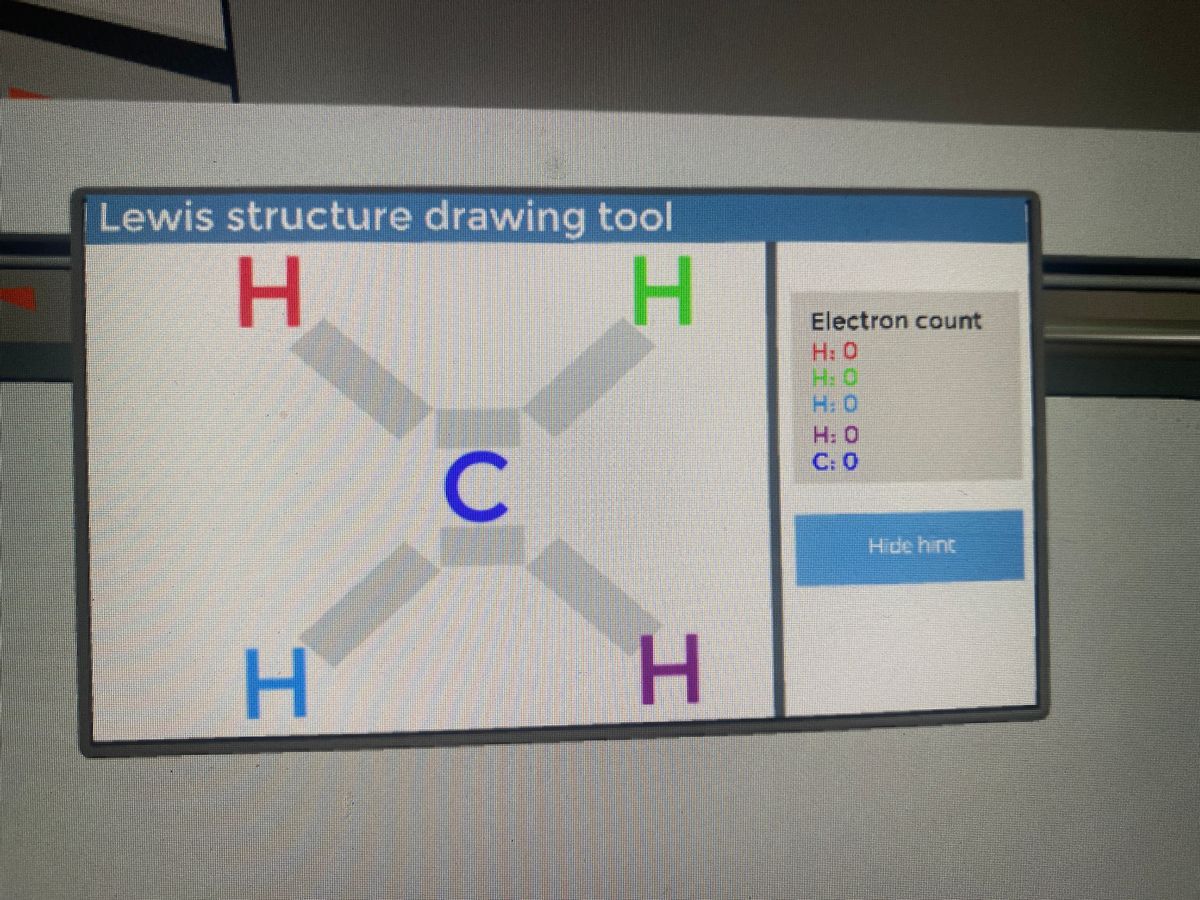
Draw Lewis dot diagram for the following. Methane (CH4 ... Diagram. Draw Lewis dot diagram for the following. Methane (CH 4) Advertisement Remove all ads.
techiescientist.com › ch2cl2-lewis-structureCH2Cl2 Lewis Structure, Molecular Geometry, Hybridization ... Mar 05, 2022 · CH2Cl2 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Dichloromethane or methylene chloride, with the chemical formula CH2Cl2, is a colorless, volatile liquid with a boiling point of 39.6 °C. and a melting point of -96.7 °C.
BeH2 Lewis Structure, Molecular Geometry, Hybridization ... 04.03.2022 · Lewis structure of the molecule is based on the concept of the octet rule. ... which is shown in the orbital diagram of the beryllium hydride molecule as follows: Hence, the VBT method also leads to the sp hybridization of the Beryllium atom in Beryllium hydride with linear geometry. BeH2 Polarity. The polarity of the covalent bond depends upon the electronegativity …
Lewis Structure of CH4 (Methane), Shape & Hybridization CH4 (methane) Lewis structure. Lewis structure is a very simplified representation of valence electrons in a chemical species like an atom, ion, or molecule. It indicates how electron are situated around the atoms either as lone pairs o bond pairs.
Solved To answer the questions, interpret the following ... To answer the questions, interpret the following Lewis diagram for CH4 H-C-H HICOH For the central carbon atom: The number of lone pairs The number of single bonds = The number of double bonds EE Submit Answer Retry Entire Group 2 more group attempts remaining
CH4 Lewis Structure, Molecular Geometry, and Hybridization ... Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Draw Lewis Structure For Ch4 - Nelson Tardwilis Molecular Orbital diagram of CH4 The molecular orbital diagram helps with determining how mixing and overlapping have taken place in a molecule to conclude upon the hybridization type. As per the figure, the four sp3 hybrid orbitals of the carbon mixes and overlaps with four 1s atomic orbitals of the hydrogen.
Nanopore Structure and Origin of Lower Permian ... CH4 adsorption in the interlayer space of Mt was supported by the lower CH4 adsorption capacity of heated Mt products (with the interlayer distance < 0.38 nm) than that of Mt at high pressures despite the higher external surface areas of the heated Mt samples. The entrance of CH4 into the interlayer space of Mt occurred at low pressures, and more CH4 mols. entered the interlayer …
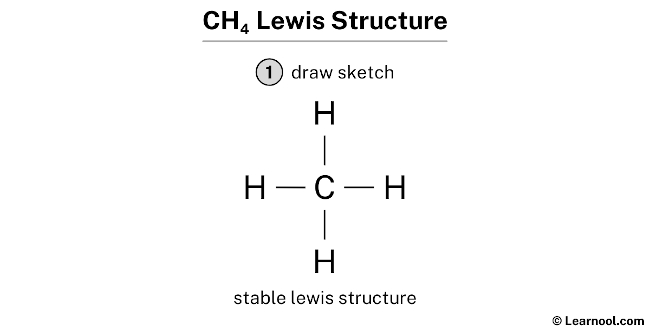
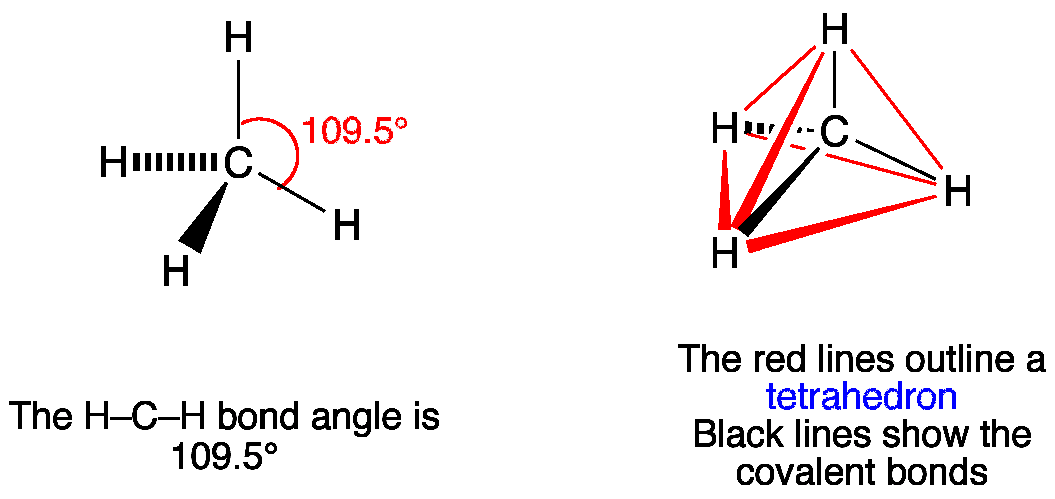
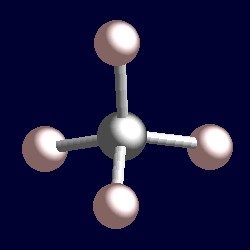
Best overview: Is CH4 Polar or Nonpolar? - Science ... The carbon atom can be the central atom in a CH4 Lewis structure diagram. As a result of this, place carbon at the center of the CH4 Lewis structure, with all four hydrogens placed around the tetrahedral geometry. Step-3: Use four single bonds (C-H) to connect the outside and core atoms in the CH4 molecule.
Lewis Dot Structure for CH4- Methane - Bob Cut Magazine Lewis Dot Structure for CH4 (Methane) Properties of methane are described by Lewis Structure as cheaper natural gas than electricity. Methane, or CH4, is a natural gas that is relatively plentiful on earth, making it an environmentally effective source.
Methane (CH4) Molecule Lewis Structure Carbon atom is the center atom and it is very easy to draw CH4 lewis structure. CH 4 lewis structure There are following specifications in the lewis structure of methane. Four hydrogen atoms have made bonds with center carbon atom and all those bonds are single bonds. No lone pairs on valence shells of carbon and hydrogen atoms.
Ch4 Electron Dot Diagram - schematron.org Drawing the Lewis structure for CH 4 (named methane) requires only single schematron.org's one of the easier Lewis structures to draw. Remember that hydrogen atoms always go on the outside of a Lewis structure and that they only need two valence electrons for a full outer shell. The Lewis Dot Structure for CH4 is shown above.
CH4 lewis structure, Molecular geometry, Polar or nonpolar ... ∴ Total valence electron available for drawing the CH4 lewis structure = 4 + 1*4 = 8 valence electrons [∴CH4 has four hydrogen atoms and one carbon atom] 2. Find the least electronegative atom and placed it at center
How to draw CH4 Lewis Structure? - Science Education and ... In a CH4 Lewis structure diagram, the carbon atom can be the centre atom. As a result, central carbon in the CH4 Lewis structure, with all four hydrogens arranged around the tetrahedral geometry. Step-3: Combining step1 and step2 to get step3 for CH4 dot structure Connect the exterior and core atoms of the CH4 molecule with four single bonds (C-H).
Lewis Structure Of Ch4 - ViralListClub.com To draw the lewis Dot structure of CH₄ methane we have to find out the valence electrons of carbon and hydrogen firstWe express valence electrons as dots in lewis dot structure. Sp 3 Then draw the 3D molecular structure using VSEPR rules. The Lewis dot structure for CH 4 shows the number of valence electrons around each atom.
Chapter 4: Covalent Bond and Lewis Structure - Chemistry 109 Ch4.5 Lewis Symbols and Lewis Structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 15 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 15.
How to Draw the Lewis Structure of CH4 (methane) - YouTube Check me out:
Ch4 Electron Dot Diagram The electrons are arranged in four pairs representing four covalent bonds. The Lewis dot structure for CH 4 shows the number of valence electrons around each atom. Each dot represents a valence electron. The number of valence electrons . Write the symbol of the atom you are drawing the electron dot diagram for in the middle of your paper.
Lewis Structure for CH4 (Methane) - UMD Drawing the Lewis Structure for CH 4 For CH 4 you have a total of 8 total valence electrons. Drawing the Lewis structure for CH 4 (named methane) requires only single bonds. It's one of the easier Lewis structures to draw.
quizlet.com › 85211460 › chemistry-final-reviewChemistry Final Review Flashcards | Quizlet The diagram below shows gas with an initial pressure of 3060 mm Hg in a cylinder at a constant temperature. The gas expands inside the Cylinder and pushes the piston up. What is the final pressure of the gas after the expansion?

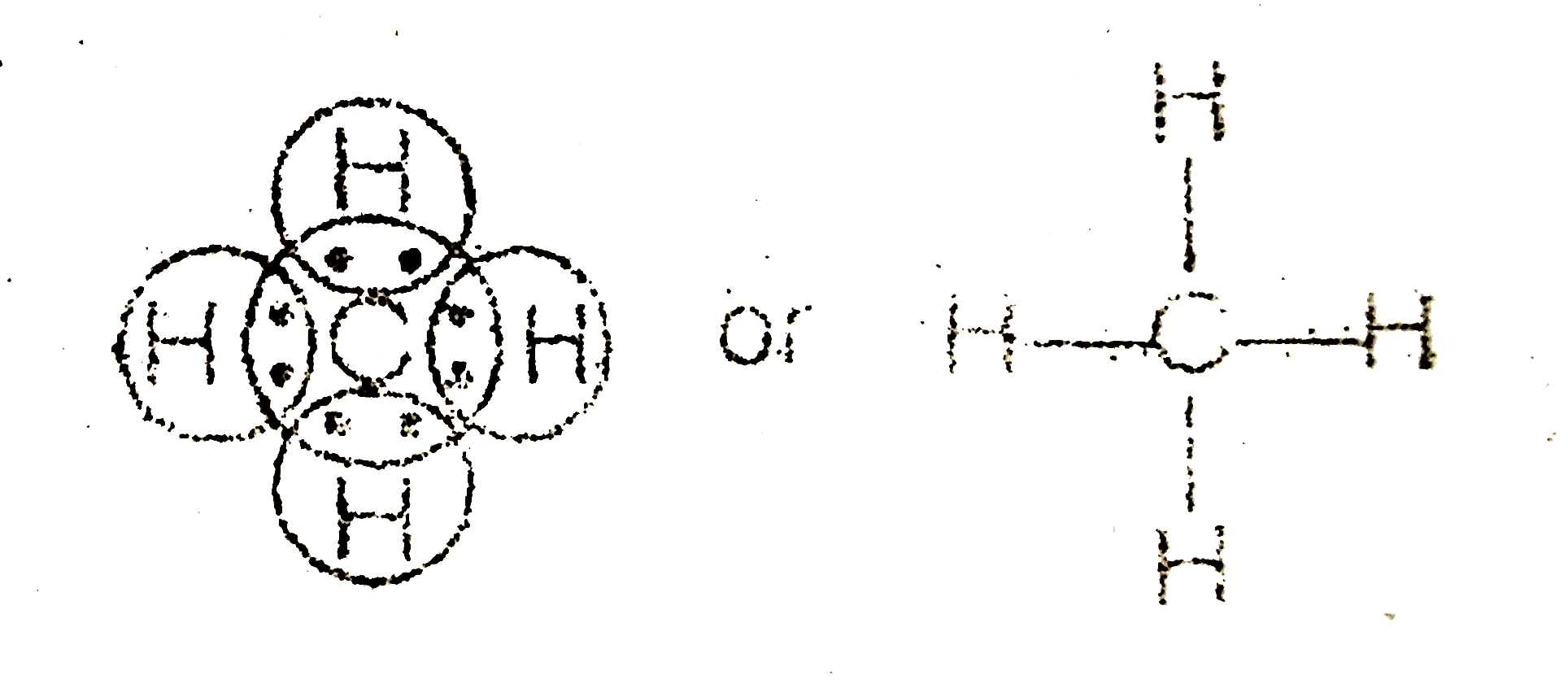
















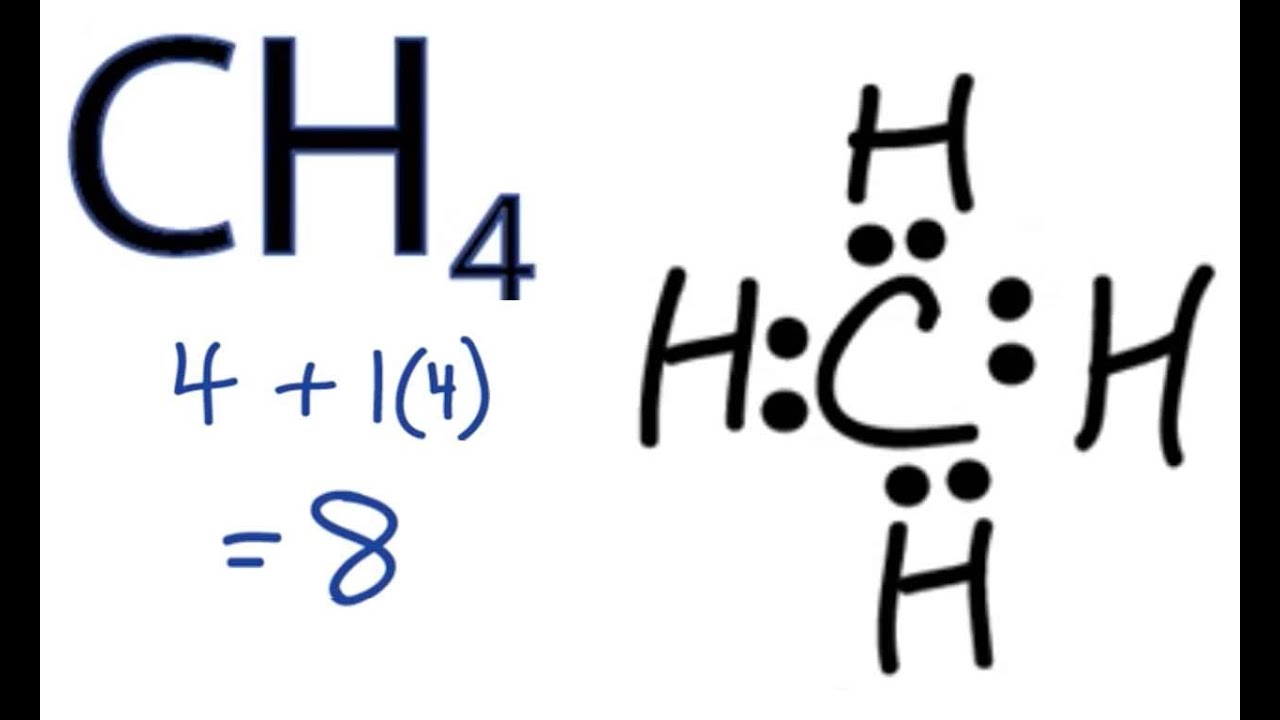










0 Response to "42 lewis diagram for ch4"
Post a Comment