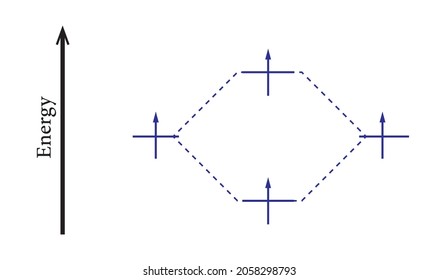
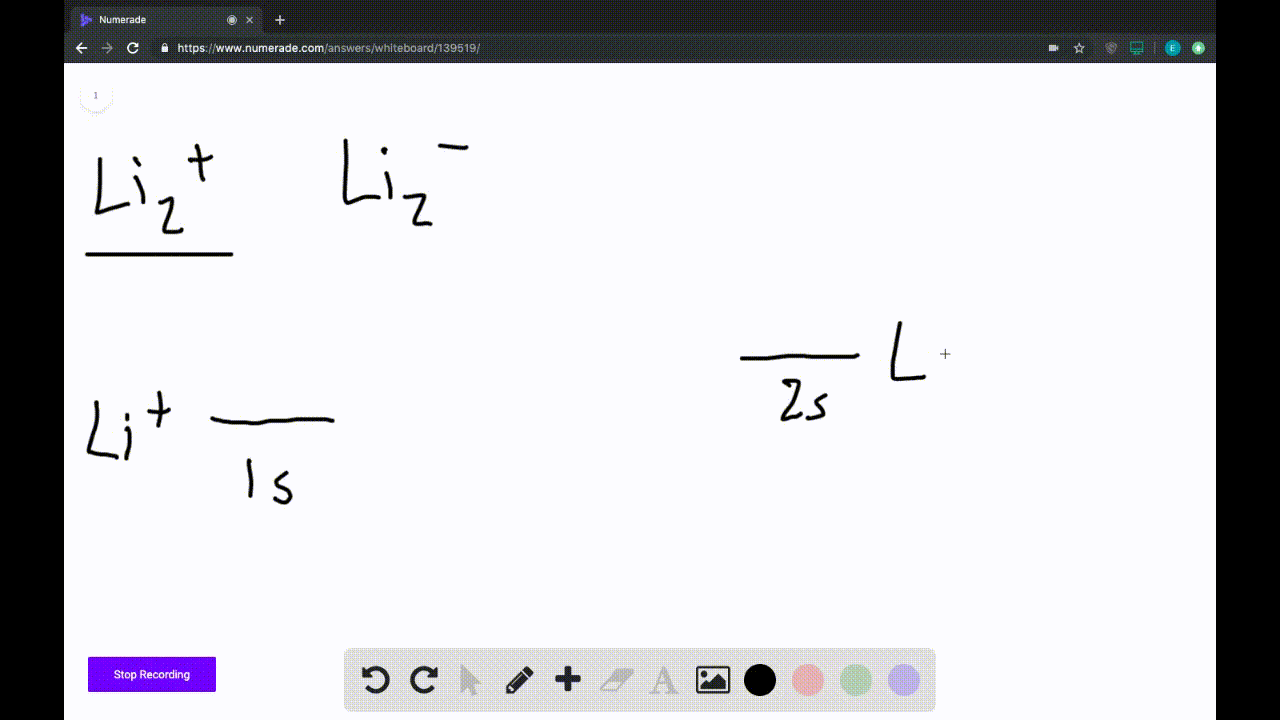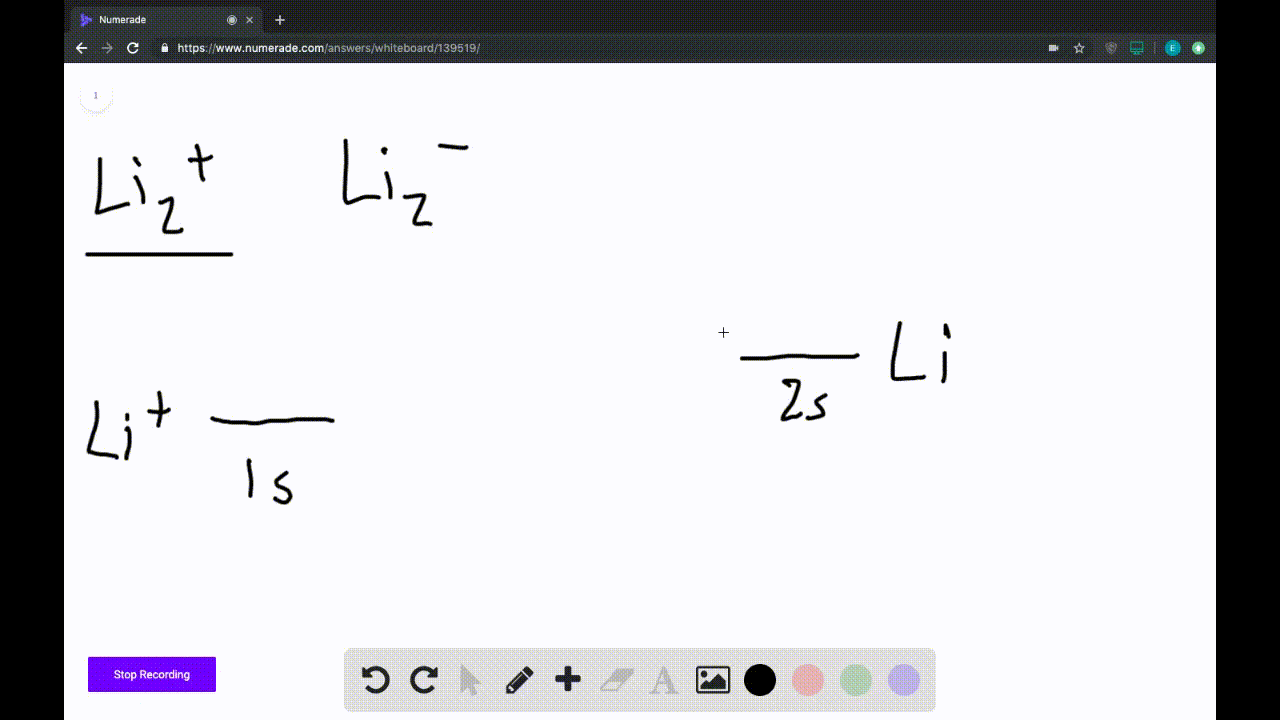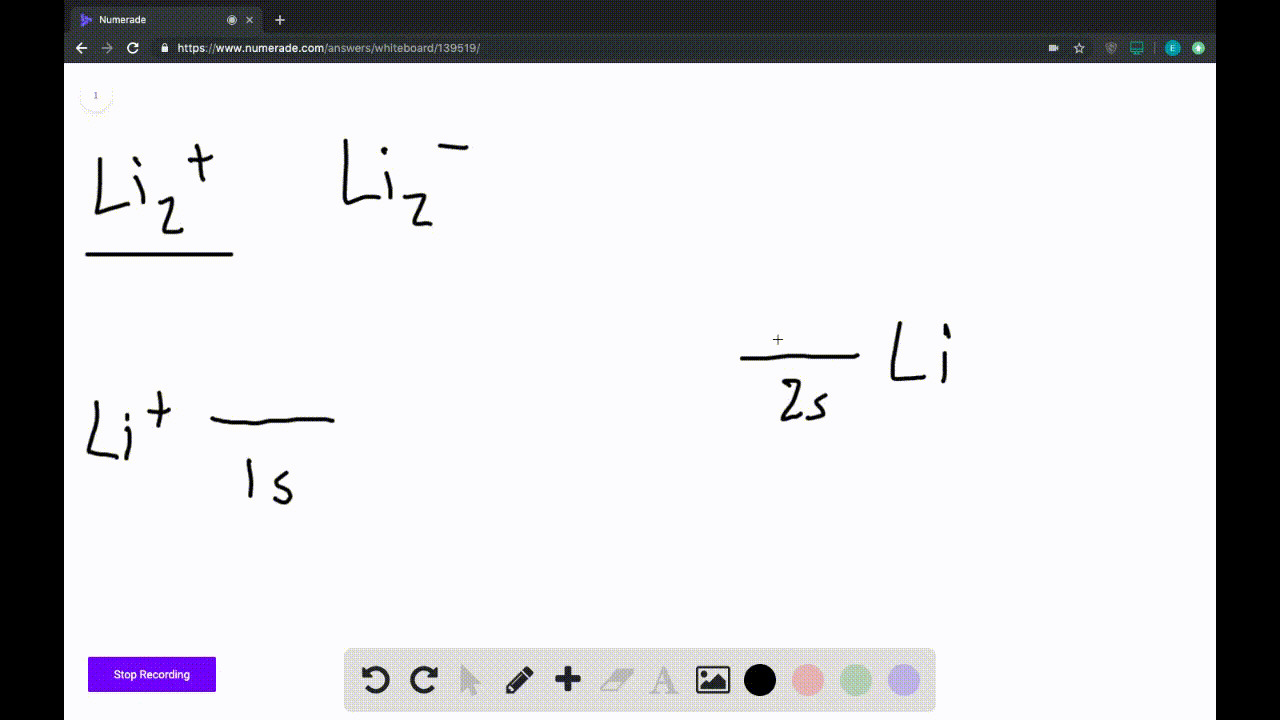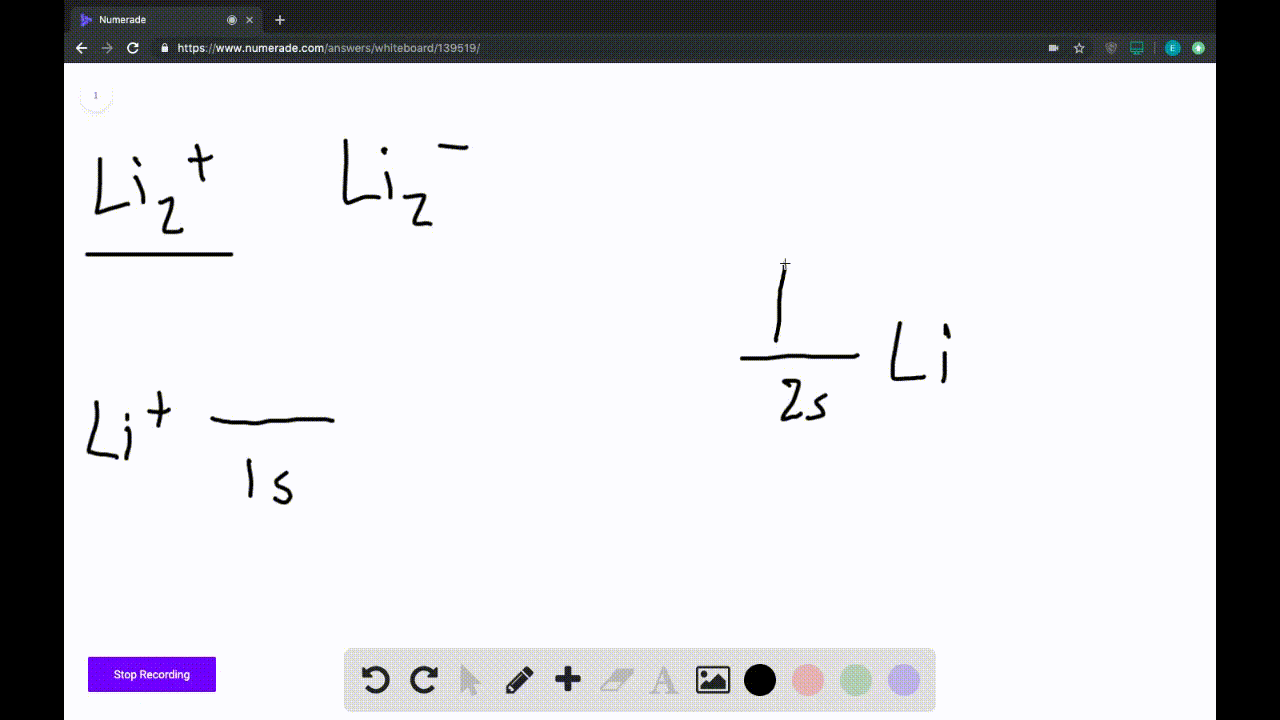
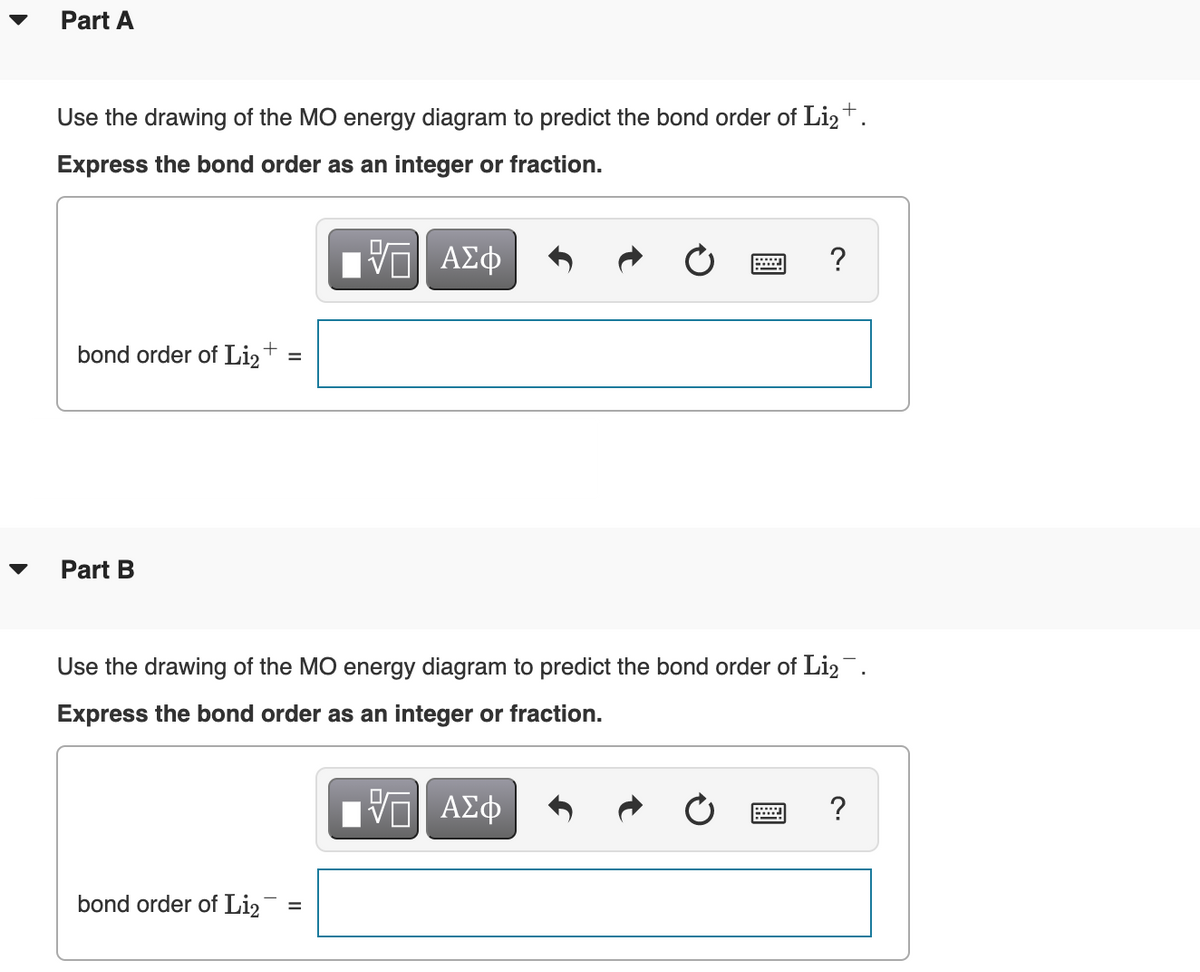
45 use the drawing of the mo energy diagram to predict the bond order of li2+
Solution for Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2. ) Q. Use an MO diagram to find the bond order and predict whether H2− exists. Q. Draw an MO energy diagram and predict the bond order of Li2+and Li2- . Do you expect these molecules to exist in the gasphase?
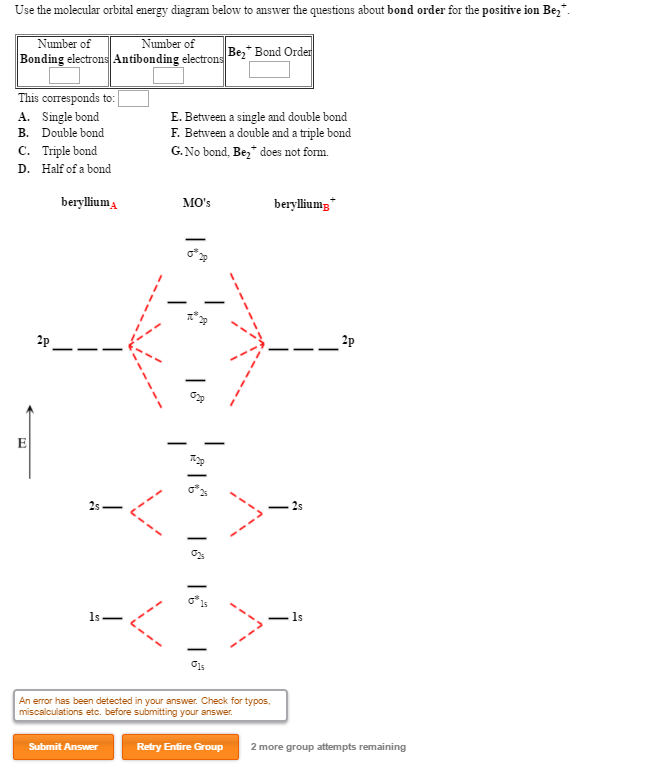
Find step-by-step Chemistry solutions and your answer to the following textbook question: Use MO diagrams and the bond orders you obtain from them to answer: (a) Is Be2+ stable? (b) Is Be2+ diamagnetic? (c) What is the outer (valence) electron configuration of Be2+?.
Use the drawing of the mo energy diagram to predict the bond order of li2+
Q. Draw the MO energy diagram for CO on your own, then use it to predict the bond order for the molecule. (Use the energy ordering of O2. (Use the energy ordering of O2. Q. Molecular nitrogen, carbon monoxide, and cyanide ion are isoelectronic. We are being asked to draw the MO energy diagram of Li 2 + and Li 2-then predict which will exist in the gas phase.. We will do the following steps. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is:. Bond Order = 1 2 [# of e- in bonding MO ... This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital.
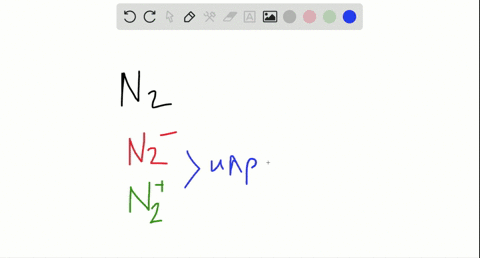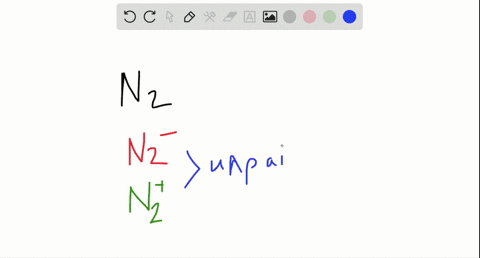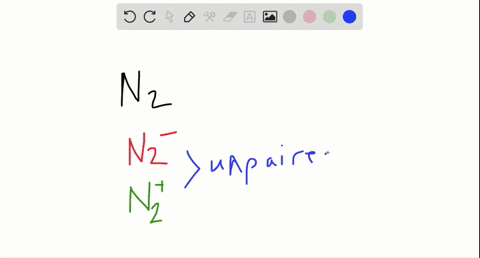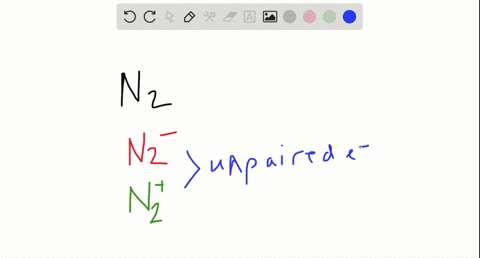
Use the drawing of the mo energy diagram to predict the bond order of li2+. This chemistry video tutorial provides a basic introduction into molecular orbital theory. It describes the formation of bonding and antibonding molecular o... Construct a "molecular orbital diagram" of the kind shown in this lesson for a simple diatomic molecule, and indicate whether the molecule or its positive and negative ions should be stable. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the ... This MO diagram depicts the molecule H2, through the contributing AOs ~ above the external sandwiching the MO. The bonding level (lower level) is totally occupied. A link order of one is acquired by use the formula above, describe a steady bond. \textBond Order = \frac2 (\textbonding electrons)-0(\textanti-bonding\ e-)2 = 1 Solution for Draw an MO energy diagram and predict the bond order of Li2 + and Li2 -. Do you expect these molecules to exist in the gas phase?
Answer to Draw a molecular orbital energy diagram for Li2. What is the bond order? Is the molecule likely to be stable? Explain. Explain why the relative energy levels diagrams for Li2, Be2, B2, C2, N2 are different The molecular orbital theory of Li2 to F2 gives a graphical explanation. MO diagrams for Diatomic Molcules. Overview. Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase? Question: Use the drawing of MO energy diagram to predict the bond order ofLi2+ and Li2- . Do youexpect Li2+ to exist in the gas phase? Use the drawing of MO energy diagram for CO to predict the bond order. (Use the energy ordering of O2. ) Express your answer using two significant figures. Best Answer. This is the best answer based on feedback and ratings. Okay, so this problem test our understanding of the molecular arbiters. They asked us to draw the molecular arbitral diagram for the elysium two plus and we see um two miners. So before we draw the diagram first we need to know the electron configurations of the issue. Alicia electron configuration we know is what has to and to s warm. So actually each lithium atom has to up tools that is ...
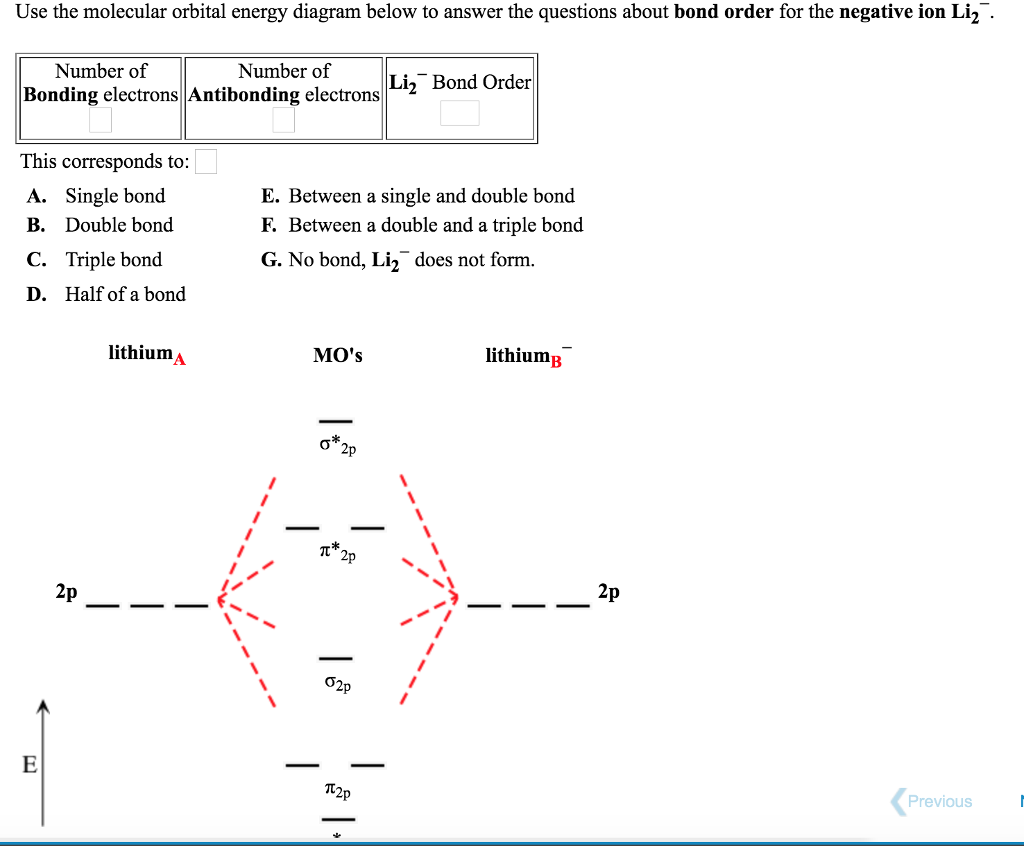
In a world where the use of magic typically means drawing power from your own body heat, a particularly crafty mage discovers a way to use a controlled nuclear fission reaction as a source of energy. What follows is an epic, action packed fight for survival. In the world of Fables, when a Fable is born a Fable becomes the very embodiment of the magic that created it. The Fable game book is available here on Amazon US, Amazon UK, Barnes and Noble US, and Barnes and Noble UK. Here's the cover: ... Express the bond order as an integer or fraction. Use the drawing of the MO energy diagram to predict the bond order of Li2−. Which molecules are predicted to exist in the gas phase? Answer to Draw an MO energy diagram and predict the bond order of Be2+ and Be2−. Do you expect these molecules to exist in the. Even rather simple molecular orbital (MO) theory can be used to predict which we start reading from the bottom of the diagram because this is how MO diagrams are constructed, Diberyllium, Be2, has a bond order of zero and is unknown. Answer to: Use the drawing of the MO energy diagram to predict the bond order of Li2+, and use the drawing of the MO energy diagram to predict the...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine
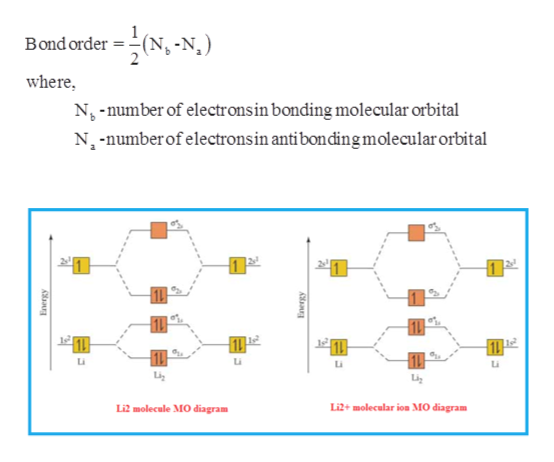
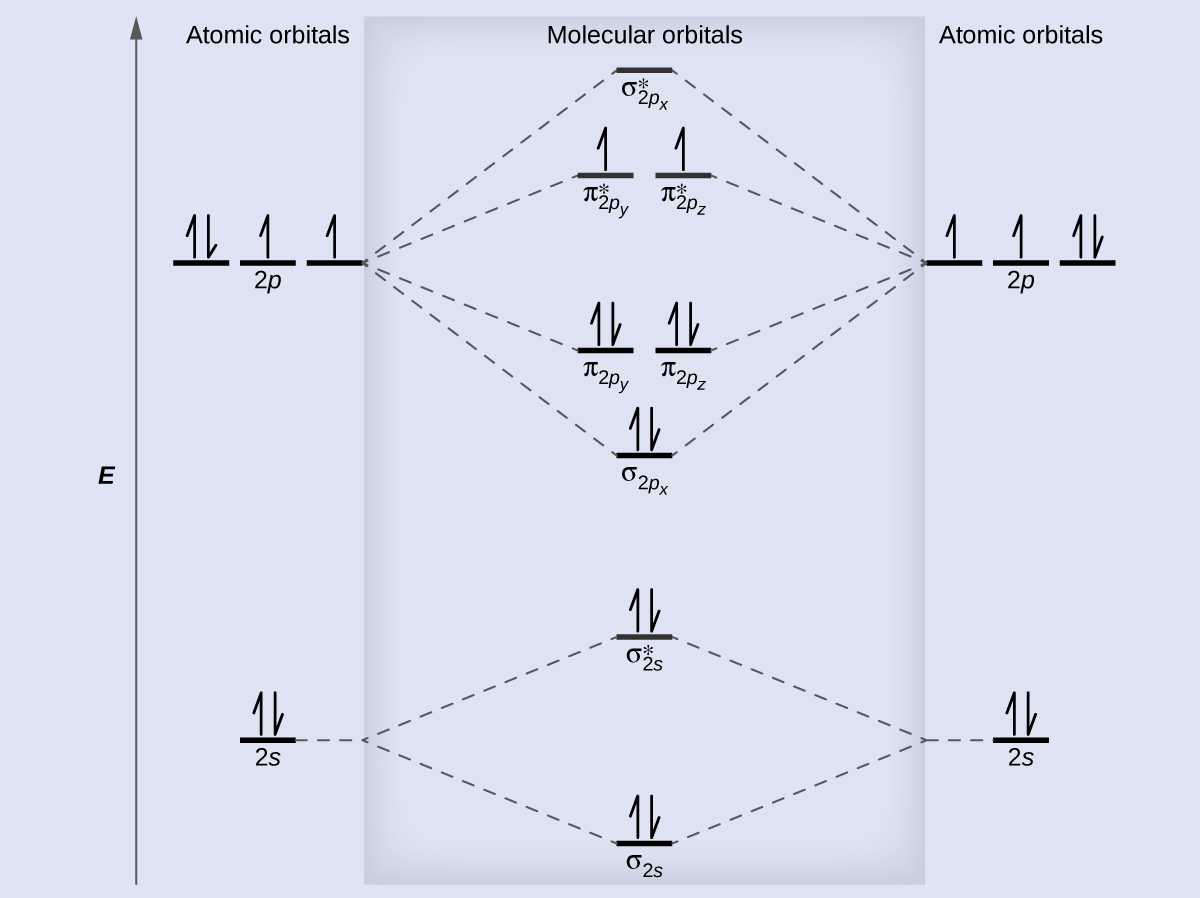
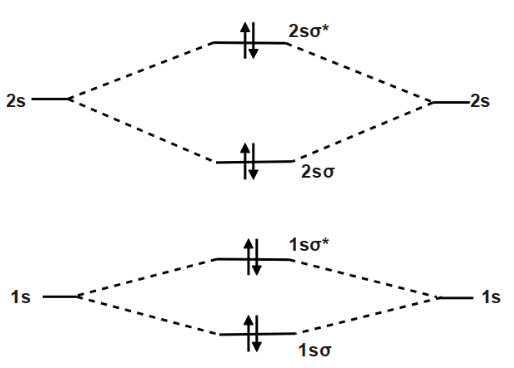
Molecular orbital energy level of Li2. diagram; the MOs go between them. Consider the MO diagram for Li2. This is a bond between two lithium atoms, which have an electron configuration of. The last diagram presents the molecule dilithium (Li2). The 1s electrons do not take part in the bonding, but the 2s electrons fill the bonding orbital.
The bond order tells us the average number of bonds between the bonded atoms. In a diatomic molecule such as `O_2`, the bond order simply tells the number of bonds between the two atoms. The bond order can be interpreted from MO diagrams using the following formula: `"Bond Order" = 1/2 [("Bonding "e^-)-("Antibonding " e^-)]`
We're being asked to determine the bond order of Li2-. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: Bond Order = 1 2 [ # of e - in ...
https://imgur.com/a/LDNi9o4 Just a bad lil drawing but remember when the strat was to put the furthest hobgoblin into status so he wouldn’t one shot the shield carrier
Anionic aluminum(I) anions (“aluminyls”) are the most recent discovery along Group 13 anions, and the understanding of the unconventional reactivity they are able to induce at a coordinated metal site is at an early stage. A striking example is the efficient insertion of carbon dioxide into the Au–Al bond of a gold–aluminyl complex. The reaction occurs via a cooperative …
Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Part A. Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B.
Li2 is more stable than Li+ 2, because the bond is (hypothetically) stronger (probably gas-phase). Here we consider the molecular orbital diagram (MO) of Li2: The bond order can be calculated in a simple manner. Just take electrons that are in each MO, and. for each electron in a bonding MO, it adds 0.5 to the bond order, because more bonding ...
Draw the MO diagram for acetylide ion C2^2- and calculate its bond order.
Use the drawing of the MO energy diagram to predict the bond order of Li2+. Express the bond order as an integer or fraction. Part B. Use the drawing of the MO energy diagram to predict the bond order of Li2?. Express the bond order as an integer or fraction. Part C. Which molecules are predicted to exist in the gas phase? Check all that apply
Except Microsoft Visio
Hi, ​ I'm struggling to understand why, in the following ML4 sigma-only complex, that the metal AO T2 set rises in energy while there is no bonding counterpart to it. ​ I appreciate the rationale in terms of crystal field theory etc but unsure how it's justified in the case of merely looking at symmetry-matching AOs and MOs. ​ [https://imgur.com/a/cNhkgjd](https://imgur.com/a/cNhkgjd) ​ Note how the T2 symmetry d-orbitals rise in energy to be a...
Modern Physics, Paul A. Tipler, Ralph Llewellyn, 6ed, Freeman, 2012
Figure 4.10.1: Molecular Orbital Energy-Level Diagrams for Homonuclear Diatomic Molecules. (a) For F 2, with 14 valence electrons (7 from each F atom), all of the energy levels except the highest, σ 2 p z ⋆ are filled. This diagram shows 8 electrons in bonding orbitals and 6 in antibonding orbitals, resulting in a bond order of 1.
Science. Chemistry. Chemistry questions and answers. Use the drawing of MO energy diagram to predict the bond order of Li2+ and Li2. Part A Do you expect Li2+ to exist in the gas phase? ANSWER: O yes O no Not completed before the time limit Part B to exist in the gas phase? Do you expect Li2 ANSWER: yes O no Not completed before the time limit.
Use the drawing of the MO energy diagram to predict the bond order of Li2+, and use the drawing of the MO energy diagram to predict the bond order of Li2−. Expert Answer. Who are the experts? Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. 100% (30 ratings)
12.01.2022 · N-Doped graphene nanoclusters (N-GNCs) are promising electrocatalysts for the oxygen reduction reaction (ORR) at the cathode of fuel cells. In this study, the dependence of the ORR activity on the size of N-GNCs was investigated using first-principles calculations based on density functional theory. The maximum electrode potential (UMax) was estimated from the …
Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
Problem: Use an MO diagram to find the bond order and predict whether H 2− exists. Problem. : Use an MO diagram to find the bond order and predict whether H 2− exists. FREE Expert Solution. Show answer. Answer: 90% (150 ratings) play-rounded-fill. play-rounded-outline.
We can therefore use a molecular orbital energy-level diagram and the calculated bond order to predict the relative stability of species such as H 2 +. With a bond order of only 1/2 the bond in H 2 + should be weaker than in the H 2 molecule, and the H-H bond should be longer.
This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular orbital.
We are being asked to draw the MO energy diagram of Li 2 + and Li 2-then predict which will exist in the gas phase.. We will do the following steps. Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is:. Bond Order = 1 2 [# of e- in bonding MO ...
Q. Draw the MO energy diagram for CO on your own, then use it to predict the bond order for the molecule. (Use the energy ordering of O2. (Use the energy ordering of O2. Q. Molecular nitrogen, carbon monoxide, and cyanide ion are isoelectronic.
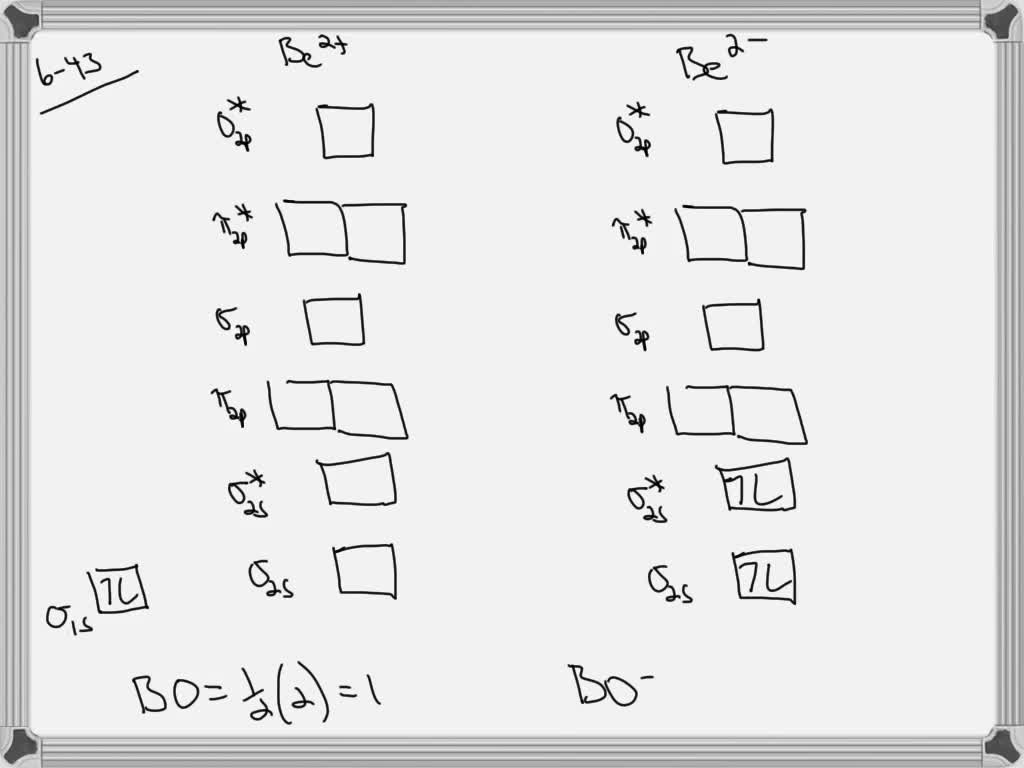
draw an mo energy diagram and predict the bond order of be2 and be2 do you expect these molecules to















![Solved] Using Figures 9.35 and 9.43 as guides, draw the ...](https://s3.amazonaws.com/si.question.images/image/images11/876-(557)-2.png)

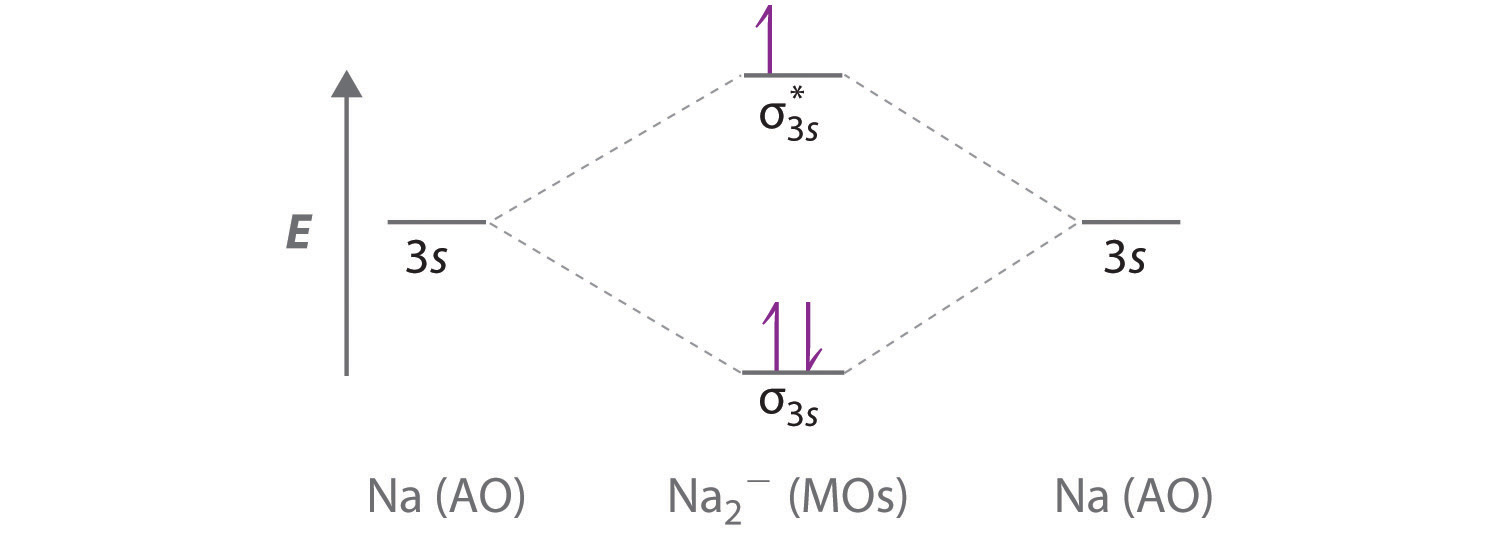


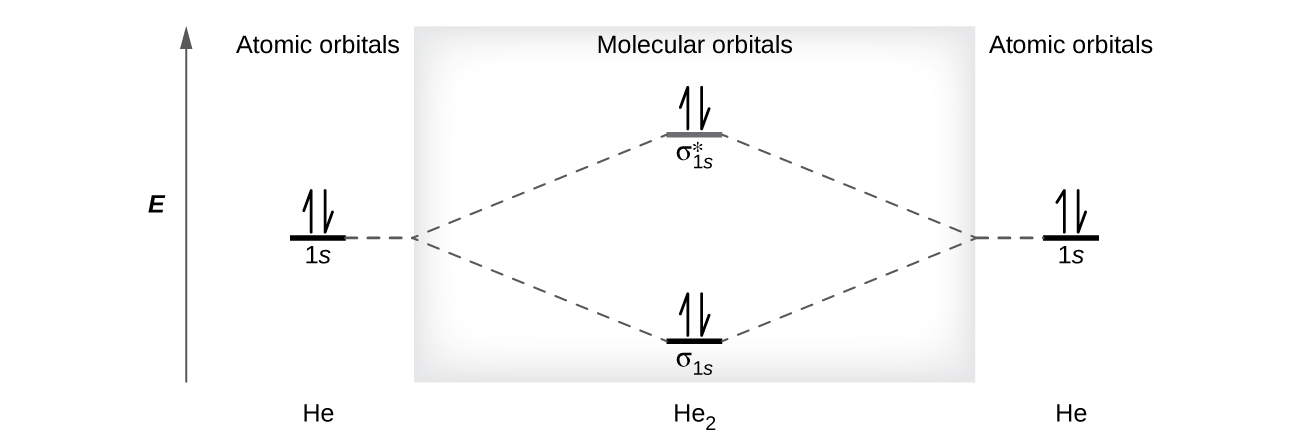
0 Response to "45 use the drawing of the mo energy diagram to predict the bond order of li2+"
Post a Comment