45 lewis dot diagram for pocl3
Lewis Structure Of Pocl3 - pocl3 lewis structure and ... Lewis Structure Of Pocl3. Here are a number of highest rated Lewis Structure Of Pocl3 pictures upon internet. We identified it from obedient source. Its submitted by dispensation in the best field. We assume this nice of Lewis Structure Of Pocl3 graphic could possibly be the most trending topic next we portion it in google help or facebook. Lewis Dot - nitrogen trifluoride nf3 lewis dot structure ... Lewis Dot. Here are a number of highest rated Lewis Dot pictures on internet. We identified it from well-behaved source. Its submitted by direction in the best field. We agree to this nice of Lewis Dot graphic could possibly be the most trending subject following we share it in google improvement or facebook.
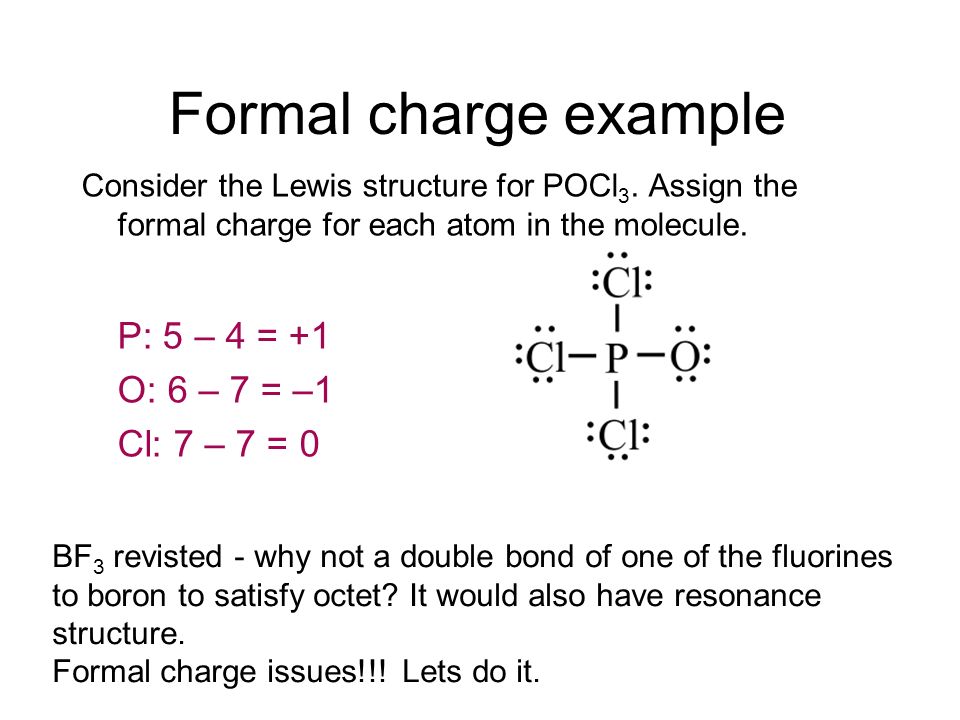
PCl3 Lewis Structure, Hybridization, Molecular Geometry ... PCl3 Lewis Structure, Hybridization, Molecular Geometry, and MO Diagram. Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. This liquid can be colorless as well. PCl3 is a toxic liquid with an unpleasant smell. The molar mass of this compound is 137.33 g/mol.

Lewis dot diagram for pocl3
POCl3 Lewis Structure - How to Draw the Lewis Structure ... A step-by-step explanation of how to draw the POCl3 Lewis Dot Structure (Phosphoryl chloride).For the POCl3 structure use the periodic table to find the tota... Phosphoryl chloride - Wikipedia Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula P O Cl 3.It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride.It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate How to Draw Lewis Structure for POCl3 phosphoryl chloride ... How to Draw a Lewis Structure for POCl3? Lewis Structure: : https...
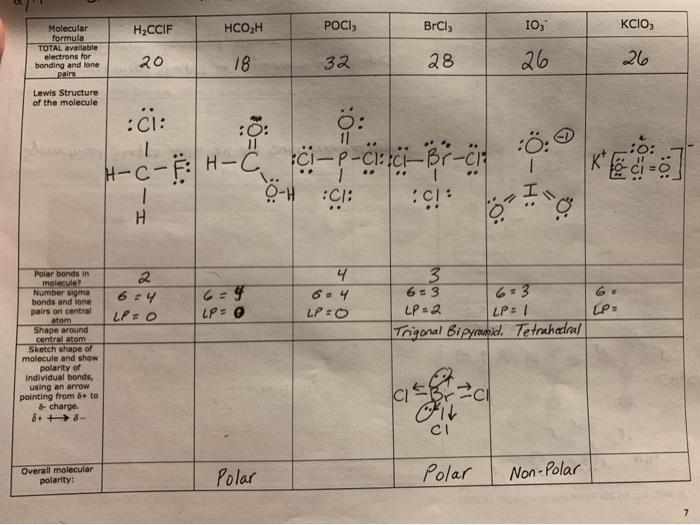
Lewis dot diagram for pocl3. Chapter 10 Practice Test Flashcards by Irina ... - Brainscape Use the Lewis Dot structure for POCl3 to calculate the SN value for the central phosphorous atom. 4 2 The Lewis Dot structure for water can be drawn in the following manner. What is the steric number value and predicted geometric shape of water based on the SN value? 4; tetrahedral 3 POCl3 Lewis Structure, Molecular Geometry, Hybridization ... POCl3 Lewis Structure. The Lewis structure of any molecule helps understand the arrangement of atoms in the molecule, its bond formation, and the valence electrons participating in forming bonds. The valence electrons that take part in forming bonds are called bonding pairs of electrons, whereas those that do not form bonds are called lone ... PCl3 (Phosphorus Trichloride) Lewis Structure PCl 3 (Phosphorus Trichloride) Lewis Structure. Phosphorus trichloride (PCl 3) contains three chlorine atoms and one phosphorus atoms. In PCl 3 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond. Also, there is a lone pair on phosphorus atom. In this tutorial, we will learn how to draw the lewis structure of PCl 3 with all theories. 7.3 Lewis Symbols and Structures - Chemistry In the Lewis structures listed here, M and X represent various elements in the third period of the periodic table. Write the formula of each compound using the chemical symbols of each element: (a) (b) (c) (d) Write the Lewis structure for the diatomic molecule P 2, an unstable form of phosphorus found in high-temperature phosphorus vapor.
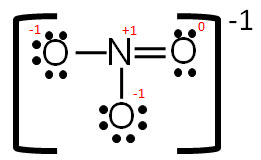
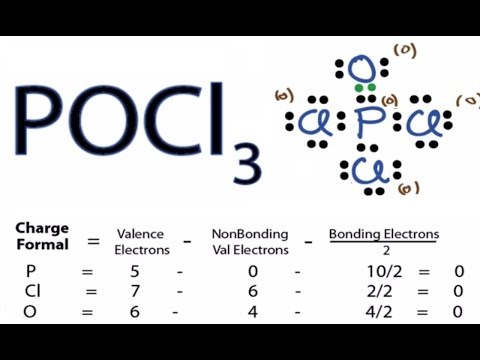
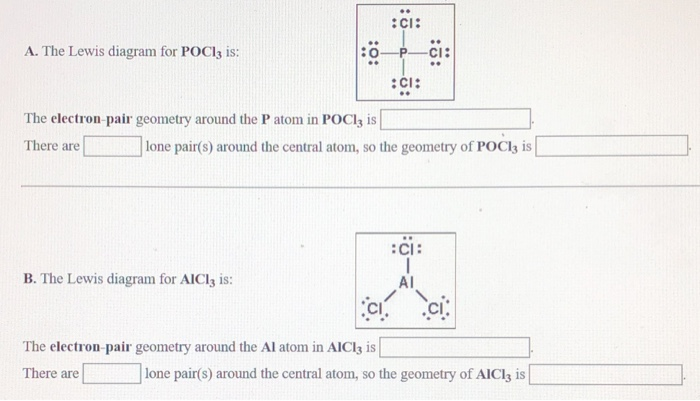
HEY MATE Q lewis dot structure of POCL3 - Brainly.in lewis dot structure of POCL3 2 See answers risheshshukla12 risheshshukla12 Answer: Mark Brainliest and Follow me . prathmeshshreypch9ul prathmeshshreypch9ul Answer: Step 1: Connect the atoms with single bonds. The less electronegative is the phosphorous atom. Hence, the P atom is going to be the central atom. Lewis Structure of POCL3? - CHEMISTRY COMMUNITY Re: Lewis Structure of POCL3? You would have to leave the P-O bond as a double bond with two lone pair electrons because when you calculate the formal charge, it would be zero at every atom. If you left it as a single bond with three lone pairs, Oxygen would have a FC of -1 and P would have a formal charge of 1. NOCl Lewis Structure, Molecular Geometry, Hybridization ... It is also known as the electron-dot structure. Lewis Structure of NOCl. Step 1: First take a molecule of NOCl. A nitrosyl chloride molecule consists of one atom of nitrogen, one atom of chlorine, and one atom of oxygen. Step 2: Now, we will find out the total number of valence electrons present in one NOCl molecule. Solved Phosphoryl Chloride, POCl3, is a reagent that is ... Question: Phosphoryl Chloride, POCl3, is a reagent that is used to make phosphate triesters from alcohols. (A) Draw a Lewis Dot structure that satisfies the octet rule for each atom in POCl3 then calculate the formal charge for each atom in the structure. (B) Draw a resonance structure of POCl3 in which all the atoms have a formal charge of zero.
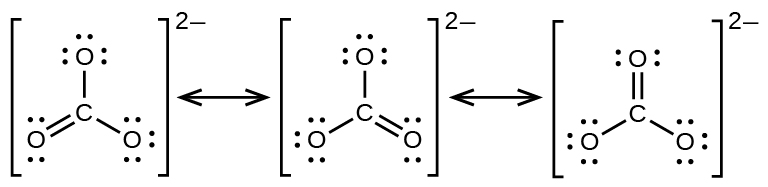
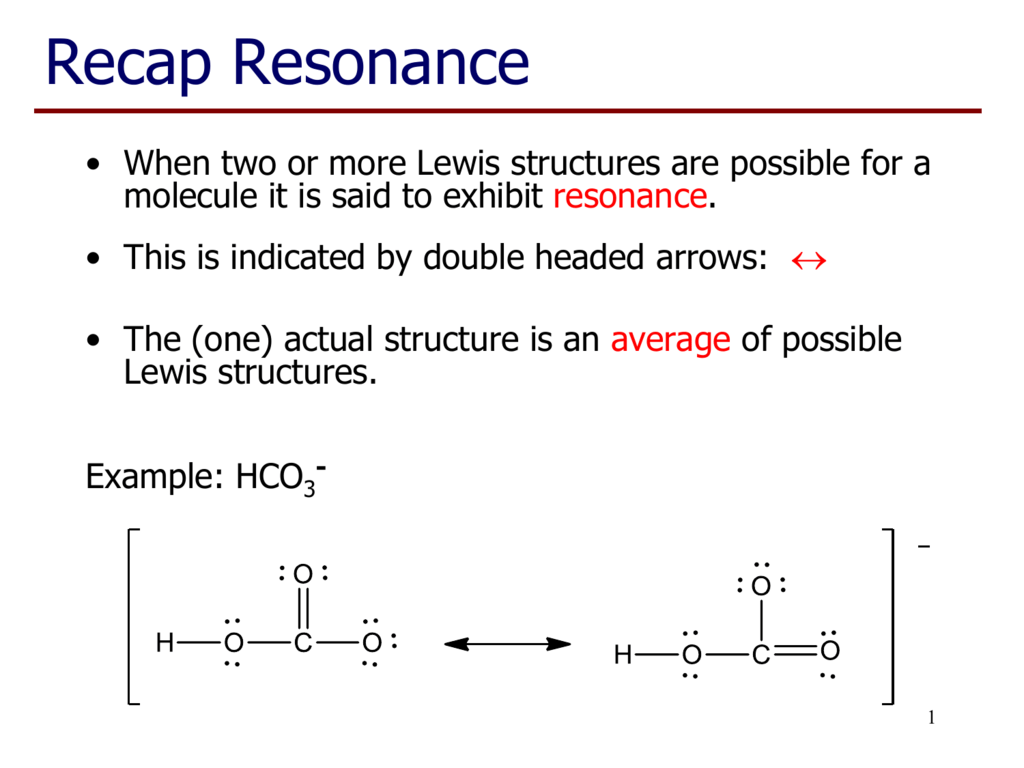
PDF Chapter 1,2 Carbon Compounds, Chemical Bonds, Lewis ... Lewis structure. For example, two valid Lewis structures can be drawn for the anion (HCONH)!. One structure has a negatively charged N atom and a C-O double bond; the other has a negatively charged O atom and a C-N double bond. These structures are called resonance structures or resonance forms. Carbon dioxide (CO2) lewis dot structure, molecular ... CO2 lewis structure contains two oxygen atoms and one carbon atom, connected with the double bond whereas carbon is the central atom, and no lone pair is present on it. But each oxygen in the CO2 lewis dot structure has two lone pairs. A lewis diagram helps us to know how electrons are arranged around individual atoms in a molecule. Lewis Structure For Pcl3 - DiviNewsMedia.com The molecular geometry of PCl 3 is trigonal pyramidal with asymmetric charge distribution on the central atom. One is phosphorous P and the second is chlorine Cl. These Atoms can exceed 8 surroundings electrons in the structure. Click and drag the molecle to rotate it. In the Lewis structure for PCl 3 there are a total of 26 valence electrons. OneClass: Phosphoryl chloride, POCl3, has the skeleton ... Get the detailed answer: Phosphoryl chloride, POCl3, has the skeleton structure. Write (a) A Lewis structure for POCl3 following the octet rule. Calculate
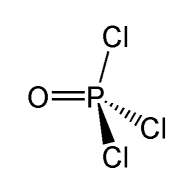
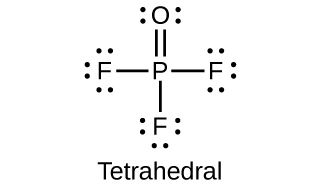
POCl3 (Phosphorus Oxychloride): Structure, Properties, & Uses Structure of POCl 3. POCl 3 molecules feature 3 phosphorus-chlorine single bonds and one phosphorus-oxygen double bond. This molecule assumes a tetrahedral shape. The structure of a POCl 3 molecule is illustrated below. Structure of POCl3. It can be noted that the P=O bond is much stronger than the P-Cl bond.
What is the Lewis dot structure for PCl3? - handlebar ... Lewis-dot structure is defined as the structure which represents the number of valence electrons around the atoms. The electrons are represented as dots. Fluorine needs 1 electron to complete its octet. When another fluorine combines, they share 1 electron each forming a single bond.
What is the Lewis structure for SeOF2? - Answers This is the Lewis Dot Structure . this may be the Lewis Structure But i am not 100% :S: ll :S: What is the Lewis structure of AsF5? I think it's similar to the Lewis structure for PCl5. So, if you ...
PCl3 Molecular Electron Geometry, Lewis Structure, Bond ... Lewis Structure. PCl3 Molecular Electron Geometry, Lewis Structure, Bond Angles and Hybridization. July 23, 2021; Posted by Priyanka; 07 Feb Phosphorus trichloride is made up of one Phosphorus atom and three Chlorine atoms, having a chemical formula of PCl3. It is a volatile liquid that reacts with water and releases HCl gas.
Lewis Structures ... 100+ Lewis Structures - The Geoexchange Every chemistry student has to learn how to draw Lewis Dot Structures. The key is to understand the steps and practice. Lewis Structures are important to learn because they help us predict: the shape of a molecule. how the molecule might react with other molecules. the physical properties of the molecule (like boiling point, surface tension, etc.).
Lewis Structures - Dot Diagram, Formal Charge, Molecular ... 19. Lewis Dot Structure For NH3 - Trigonal Pyramidal - Bond Angle of 107, Sp3 Hybridized. 20. Lewis Structure For H2S - Bent Molecular Geometry and Tetrahedral Electron Pair Geometry 21. Molecular Geometry vs Electron Pair Geometry 22. Lewis Structure For SF6 - Octahedral Molecular Geometry, 90 Bond Angle, Sp3d2 Hybridized, Nonpolar 23.
POCl3 Lewis Structure: How to Draw the Dot Structure for POCl3 In the POCl 3 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. In the Lewis structure for POCl 3 there are a total of 32 valence electrons. If you check the formal charges for POCl 3 you'll find that you need a double bond between the Phosphorous and Oxygen atom in order to have the formal charges equal zero.
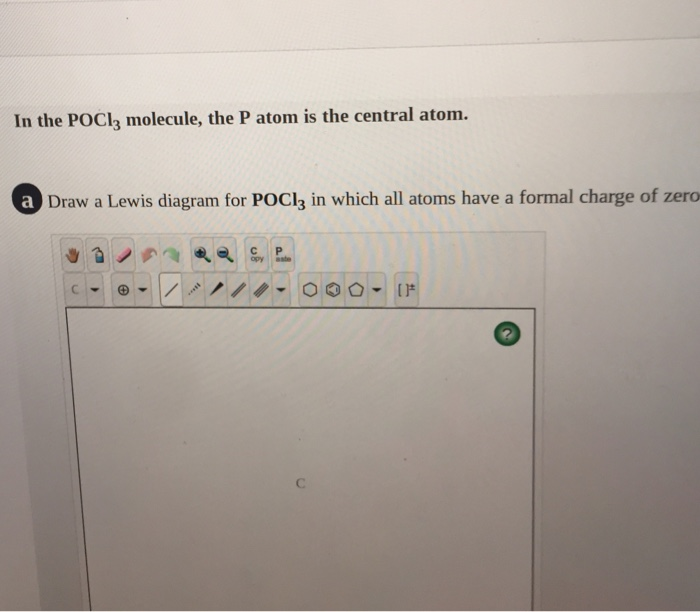
Solved In the POCl3 molecule, the P atom is the central ... Experts are tested by Chegg as specialists in their subject area. We review their content and use your feedback to keep the quality high. tha …. View the full answer. Transcribed image text: In the POCl3 molecule, the P atom is the central atom. Draw a Lewis diagram for POCl3 in which all atoms have a formal charge of zero.
Pocl3 Lewis Dot Structure - simple method for writing ... Pocl3 Lewis Dot Structure. Here are a number of highest rated Pocl3 Lewis Dot Structure pictures on internet. We identified it from reliable source. Its submitted by meting out in the best field. We put up with this kind of Pocl3 Lewis Dot Structure graphic could possibly be the most trending topic considering we allocation it in google gain or ...
Lewis Electron Dot Structures - Detailed Explanation with ... Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Write Lewis structure that obey the octet ... | Clutch Prep Write Lewis structure that obey the octet rule for the following molecules and ions. (In this case the first atom listed is the central atom.) a. POCl 3, SO 42-, XeO 4, PO 43-, ClO 4-. Learn this topic by watching Lewis Dot Structures: Ions Concept Videos.
How to Draw Lewis Structure for POCl3 phosphoryl chloride ... How to Draw a Lewis Structure for POCl3? Lewis Structure: : https...
Phosphoryl chloride - Wikipedia Phosphoryl chloride (commonly called phosphorus oxychloride) is a colourless liquid with the formula P O Cl 3.It hydrolyses in moist air releasing phosphoric acid and fumes of hydrogen chloride.It is manufactured industrially on a large scale from phosphorus trichloride and oxygen or phosphorus pentoxide. It is mainly used to make phosphate esters such as tricresyl phosphate
POCl3 Lewis Structure - How to Draw the Lewis Structure ... A step-by-step explanation of how to draw the POCl3 Lewis Dot Structure (Phosphoryl chloride).For the POCl3 structure use the periodic table to find the tota...























0 Response to "45 lewis dot diagram for pocl3"
Post a Comment