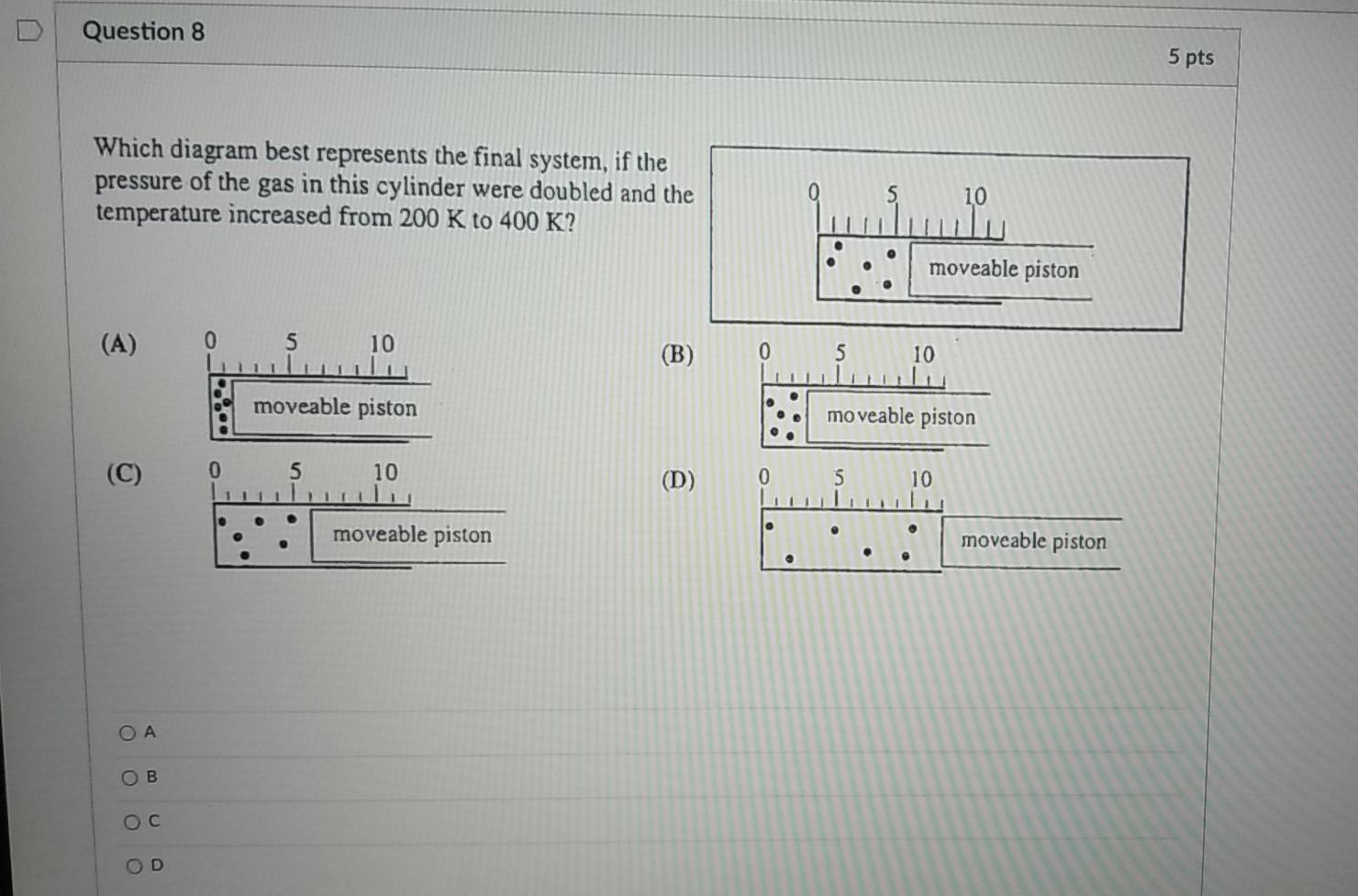
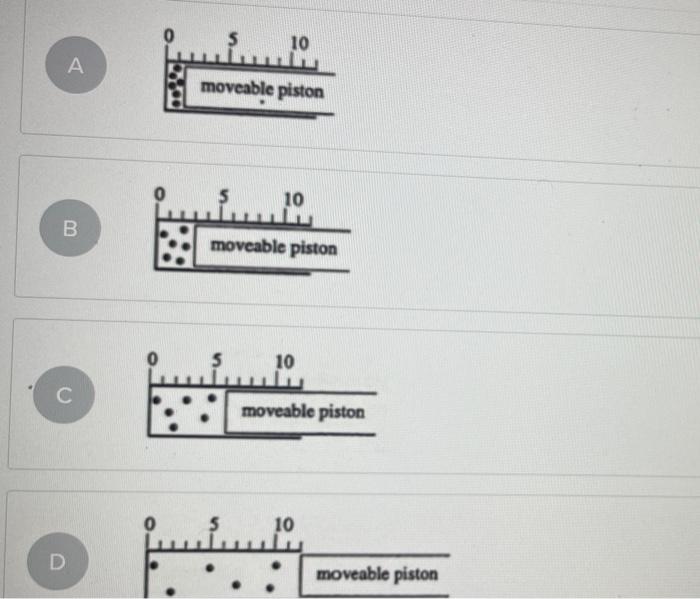
43 Which Diagram Best Represents The Final System If The Pressure Of The Gas In This Cylinder
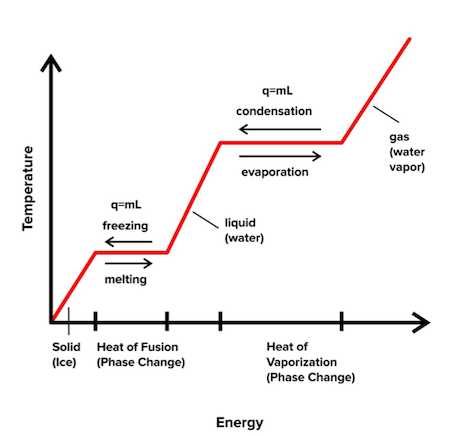
10.4 Phase Diagrams - Chemistry Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. PDF AME 20231 Homework Solutions1 Spring 2012 3. 3.122 A cylinder has a thick piston initially held by a pin as shown in Fig. P3.122. The cylinder contains carbon dioxide at 200 kPa and ambient temperature of 290 K. The metal piston has a density of 8000 kg/m3 and the atmospheric pressure is 101 kPa. The pin is now removed, allowing the piston to move and after a while the gas returns to ...
Chapter 6, Gases Video Solutions, General ... - Numerade A 4.0 L sample of $\mathrm{O}_{2}$ gas has a pressure of 1.0 bar. $\mathrm{A}$ 2.0 L sample of $\mathrm{N}_{2}$ gas has a pressure of 2.0 bar. If these two samples are mixed and then compressed in a 2.0 L vessel, what is the final pressure of the mixture? Assume that the temperature remains unchanged.
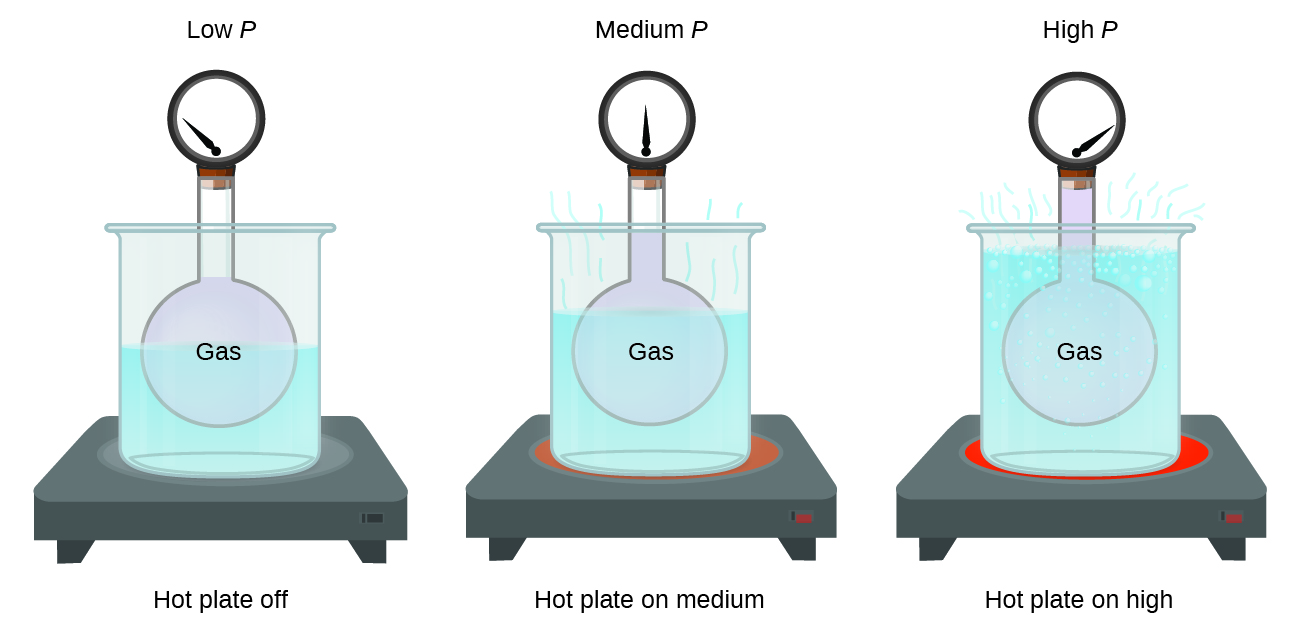
Which diagram best represents the final system if the pressure of the gas in this cylinder
PDF Example 1 - Clarkson University Discussion of Results: The process line on the P-v diagram goes from right to left, which has a negative area beneath it. A negative area on a P-v diagram indicates that work is done on the system (W IN), which is consistent with our analytic answer. Adiabatic Process - Definition, Equation, Reversible ... For instance, the gas compression within an engine cylinder is expected to happen so fast that on the compression process timescale, a minimum amount of the energy of the system could be produced and sent out in the form of heat. Despite the cylinders being not insulated and having a conductive nature, the process is deemed to be adiabatic. Phase Diagrams - Chemistry - University of Hawaiʻi Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance.
Which diagram best represents the final system if the pressure of the gas in this cylinder. PDF Chemical kinetics Name: Date - The Leon M. Goldstein High ... The accompanying diagram represents the energy changes ... Most of the propane in a cylinder is liquid, with gas in the space above the liquid level. When propane is released from the cylinder, the propane leaves the cylinder ... A. decreasing the pressure of the system B. decreasing the concentration of HCl(g) Gas Laws and KMT | Chemistry - Quizizz SURVEY. Ungraded. 180 seconds. Report an issue. Q. A 3.00-liter sample of gas is at 288 K and 1.00 atm. If the pressure of the gas is increased to 2.00 atm and its volume is decreased to 1.50 liters, the Kelvin temperature of the sample will be. answer choices. 432 K. Which diagram best represents the final system, if th... Problem: Which diagram best represents the final system, if the pressure of the gas in this cylinder were doubled and the temperature increased from 200 K to 400 K? FREE Expert Solution Show answer A system contains 0.15 𝑚3 of a gas at a pressure of 3.8 ... The diagram below represents a phase change. Which of the following best describes what is happening in the diagram? A. A liquid is changing into a gas because pressure in the system is increasing. B. A gas is changing into a . Chemistry. A 108 mL gas sample has a mass of 77.96 mg at a pressure of 1140 mmHg and a temperature of 183 °C.
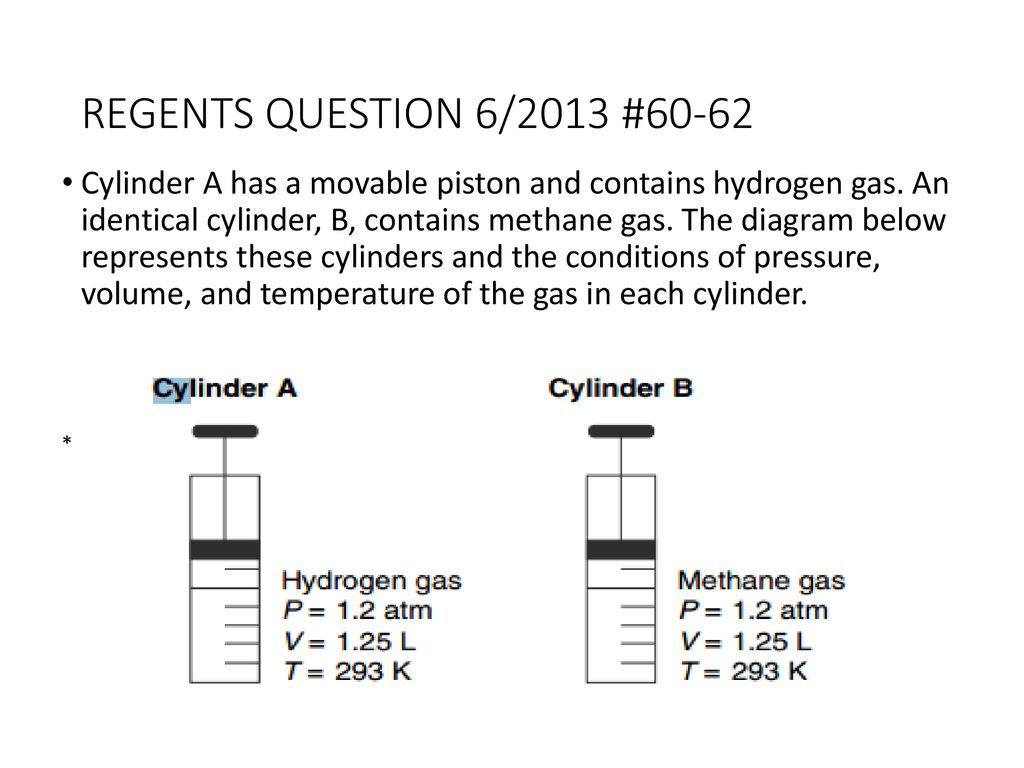
PDF Name: Gases Review Packet 2016 Cylinder A has a movable piston and contains hydrogen gas. An identical cylinder, B, contains methane gas. The diagram below represents these cylinders and the conditions of pressure, volume, and temperature of the gas in each cylinder. 27.Show a numerical setup for calculating the volume of the gas in cylinder B at STP. PDF 8th Grade Practice Final - Mr. Rohm's Science Class The mass of the object could best be determined by 1) using a balance 2) placing it in a beaker of water ... The diagram at the right represents a cylinder which contains four different liquids, W, X, Y, and Z, each with a ... The map below shows a low-pressure system located over an area in the south-central United States. 9.2 Relating Pressure, Volume, Amount, and Temperature ... Calculate the mass of the gas remaining in the cylinder if it were opened and the gas escaped until the pressure in the cylinder was equal to the atmospheric pressure, 0.983 atm, and a temperature of 27 °C. While resting, the average 70-kg human male consumes 14 L of pure O 2 per hour at 25 °C and 100 kPa. PDF Regents review Physical properties of matter 2011-2012 40. The diagram below represents a gas confined in a cylinder fitted with a movable piston. A) P + V = k B) P - V = k C) P V = k D) P × V = k As the piston moves toward point A at constant temperature, which relationship involving pressure (P) and volume (V) is correct? A) 120 L B) 20. L C) 30. L D) 40. L 41.If 60. liters of hydrogen gas at ...
[Solved] A gaseous sample of a substance is ... - SolutionInn Consider the following gaseous sample in a cylinder fitted with a movable piston. Initially there are n moles of the gas at temperature T, pressure P, and volume V. Choose the cylinder that correctly represents the gas after each of the following changes. (1) The pressure on the piston is tripled... PDF Chapter 3 Water contained in a piston-cylinder assembly undergoes two processes in series from an initial state where the pressure is 10 bar (1 MPa) and the temperature is 400oC. Process 1-2: The water is cooled as it is compressed at a constant pressure of 10 bar to the saturated vapor state. Process 2-3: The water is cooled at constant volume to 150oC. PDF Chapter 5: The First Law of Thermodynamics: Closed Systems A gas in piston-cylinder assembly undergoes a polytropic expansion. The initial pressure is 3 bar, the initial volume is 0.1 m3, and the final volume is 0.2 m3. Determine the work for the process, in kJ, if a) n=1.5, b) n=1.0, and c) n=0. Solution: Assume that i) the gas is a closed system, ii) the moving boundary is only work PDF Name Gas Law Review Date - Welcome to Dr. Mintz's ... gas. The diagram below represents these cylinders and the conditions of pressure, volume, and temperature of the gas in each cylinder. 36.Show a numerical setup for calculating the volume of the gas in cylinder B at STP. 37.State a change in temperature and a change in pressure that will cause the gas in cylinder A to behave more like an ideal gas.
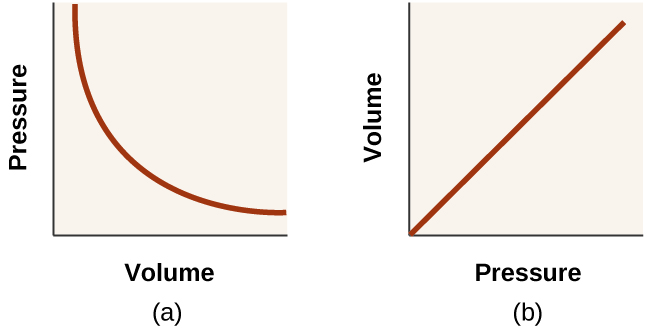
Pressure-Volume Diagram: Definition & Example - Study.com Pressure and Volume. Pressure is force (in newtons, N) divided by the area ( in square meters, m 2) over which it acts (P = F/A). Volume is the amount of space is taken up by an object or, in our ...
A sample of gas has an initial volume of 13.9 L at a ... Which diagram best represents the final system, if the pressure of the gas in this cylinder were doubled and the temperature increased from 200 K to... Q. If 0.300 moles of nitrogen gas occupies 0.600 L under certain conditions, what number of molesoccupies 1.500 L under the same conditions?
Solved Which diagram best represents the final system, if ... Which diagram best represents the final system, if the pressure of the gas in this cylinder were doubled and the temperature increased from 200 K to 400 K? Question: Which diagram best represents the final system, if the pressure of the gas in this cylinder were doubled and the temperature increased from 200 K to 400 K?
Solved Which diagram best represents the final system, if ... Chemistry. Chemistry questions and answers. Which diagram best represents the final system, if the pressure of the gas in this cylinder were doubled and the temperature increased from 200 K to 400 K? moveable piston (A) 10 (B) 10 moveable piston moveable piston 10 0 5 (C) (D) 10 moveable piston moveable piston A А moveable piston 10 B moveable ...
The pressure in the gas contained in a piston-cylinder The pressure in the gas contained in a piston-cylinder arrangement changes according to P = a + 30/V where P is in psi and V is in ft3. Initially the pressure is 7 psia and the volume is 3 ft3. Determine the work done if the final pressure is 50 psia. Show the area that represents the work on a P-V diagram.
Chem 106 Final Flashcards | Quizlet Which diagram best represents the final system, if the pressure of the gas in this cylinder were doubled and the temperature increased from 200K to 400K? see images. C. An open-ended mercury manometer is used to measure the pressure exerted by a trapped gas as shown in the figure. Atmospheric pressure is 749 mmHg.
PDF Name: Unit 8: Gases Exam - Mr. Palermo's Flipped Chemistry ... Cylinder A has a movable piston and contains hydrogen gas. An identical cylinder, B, contains methane gas. The diagram below represents these cylinders and the conditions of pressure, volume, and temperature of the gas in each cylinder. 16.Show a numerical setup for calculating the volume of the gas in cylinder B at STP.
2 Almost fully-resisted expansion of a gas in a piston ... Download scientific diagram | 2 Almost fully-resisted expansion of a gas in a piston-cylinder device. from publication: Thermodynamics for Beginners - Chapter 7 BOUNDARY WORK | In this chapter we ...
Unit 8 exercise 7 - ANSWER KEY - Hendrick Hudson Central ... below shows the initial temperature, pressure, and volume of the gas sample, as well as the final temperature and pressure of the sample. IWium Gas in a Closed System Temperature Pressure Volume Condition (atm) (mL) 2.0 final 14. Show a correct numerical setup for the final volume of the helium gas sample. & do 5.
Physics Test Review Flashcards | Quizlet The gas is initially in a state with pressure Pi, temperature Ti, and volume Vi. The piston is slowly compressed and data are recorded until the gas reaches a final state with pressure Pf, temperature Tf, and volume Vf. A graph of the pressure as a function of volume is shown, with dotted lines indicating isotherms.
PDF Name: Date: Chemistry Gas Laws 2017 Mrs. Mannion The diagram and data below represent a gas and the conditions of pressure, volume, and temperature of the gas in a rigid cylinder with a moveable piston. 24.State one change in temperature and one change in pressure that will cause the gas in the cylinder to behave more like an ideal gas. 25.Determine the volume of the gas in the cylinder at STP.
Phase Diagrams - Chemistry - University of Hawaiʻi Making such measurements over a wide range of pressures yields data that may be presented graphically as a phase diagram. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance.
Adiabatic Process - Definition, Equation, Reversible ... For instance, the gas compression within an engine cylinder is expected to happen so fast that on the compression process timescale, a minimum amount of the energy of the system could be produced and sent out in the form of heat. Despite the cylinders being not insulated and having a conductive nature, the process is deemed to be adiabatic.
PDF Example 1 - Clarkson University Discussion of Results: The process line on the P-v diagram goes from right to left, which has a negative area beneath it. A negative area on a P-v diagram indicates that work is done on the system (W IN), which is consistent with our analytic answer.











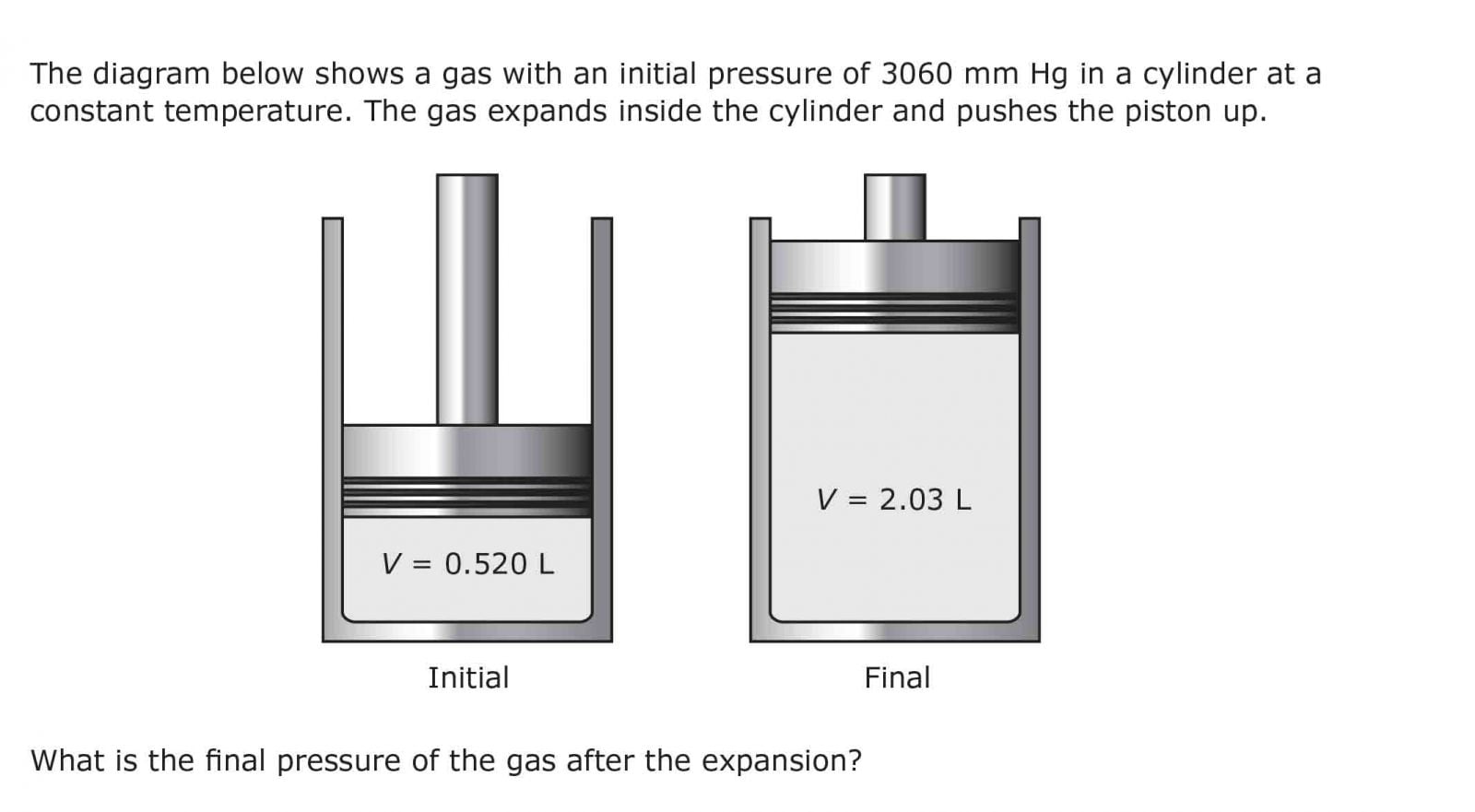

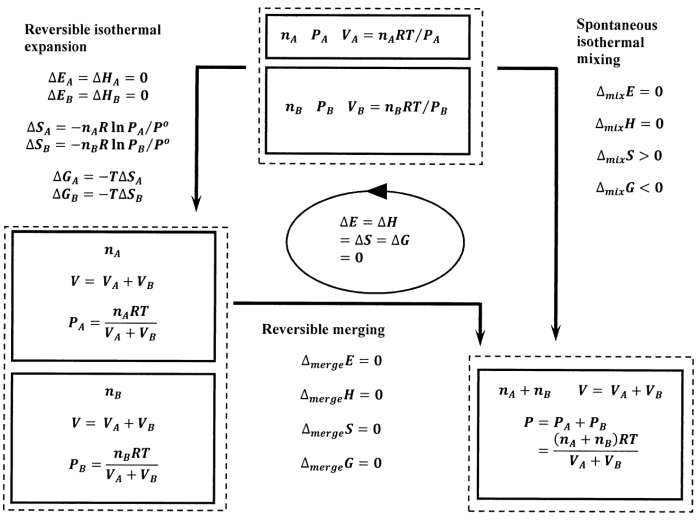



0 Response to "43 Which Diagram Best Represents The Final System If The Pressure Of The Gas In This Cylinder"
Post a Comment