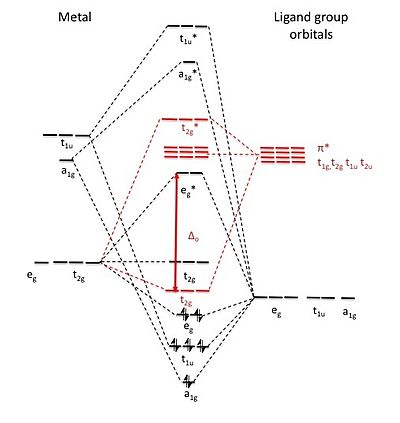
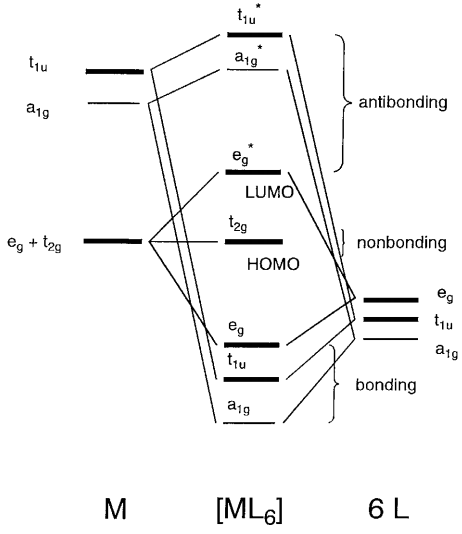
42 pi acceptor mo diagram
Orbital hybridisation - Wikipedia In chemistry, orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory.For example, in a carbon atom which forms four single bonds the valence-shell s orbital combines with three ... Custom Scholars – Your ultimate homework help service Cheap essay writing sercice. If you need professional help with completing any kind of homework, Custom Scholars is the right place to get it. Whether you are looking for essay, coursework, research, or term paper help, or with any other assignments, it is no problem for us.
Strategies on improving the electrocatalytic hydrogen ... 01.11.2021 · Previously, we synthesized Mo phosphides supported by different carbon matrices and demonstrated the high HER performances of these catalysts [58, 59]. However, the related synthesis is usually performed at high temperatures and is therefore energy-consuming and prone to induce the clumping of nanoparticles into irregular aggregates, which limits the number of …
Pi acceptor mo diagram
mini-lis.de Vor 2 Tagen · email protected] [email protected] = V0 [email protected] [email protected],USM EEE105: CIRCUIT THEORY 188 7. The code is done based on the basic differential equations and also on the formulas relating to the series ad parallel circuit of RLC. Achiever Student: We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations. (PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
Pi acceptor mo diagram. Activating and Deactivating Groups In Electrophilic ... 26.09.2017 · As you may already know, the opposite of a “pi-donor” is a “pi acceptor”. Certain functional groups can accept, rather than donate, a pi bond from the ring, resulting in a new lone pair on a substituent atom. Examples are NO 2, carbonyl groups (C=O), sulfonyl, cyano (CN) among others. These groups are universally deactivating, slowing the rate of electrophilic … (PDF) Inorganic Chemistry Housecroft | Yurika Almanda ... Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link. Fountain Essays - Your grades could look better! Your grades could look better! All our academic papers are written from scratch. All our clients are privileged to have all their academic papers written from scratch. Double bond - Wikipedia With 133 pm, the ethylene C=C bond length is shorter than the C−C length in ethane with 154 pm. The double bond is also stronger, 636 kJ mol −1 versus 368 kJ mol −1 but not twice as much as the pi-bond is weaker than the sigma bond due to less effective pi-overlap. In an alternative representation, the double bond results from two overlapping sp 3 orbitals as in a bent bond.
(PDF) Inorganic Chemistry by Miessler ~ 5th Edition ... This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Achiever Student: We always make sure that writers follow all your instructions precisely. You can choose your academic level: high school, college/university, master's or pHD, and we will assign you a writer who can satisfactorily meet your professor's expectations. mini-lis.de Vor 2 Tagen · email protected] [email protected] = V0 [email protected] [email protected],USM EEE105: CIRCUIT THEORY 188 7. The code is done based on the basic differential equations and also on the formulas relating to the series ad parallel circuit of RLC.




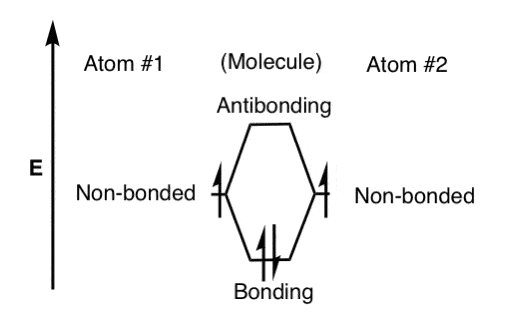
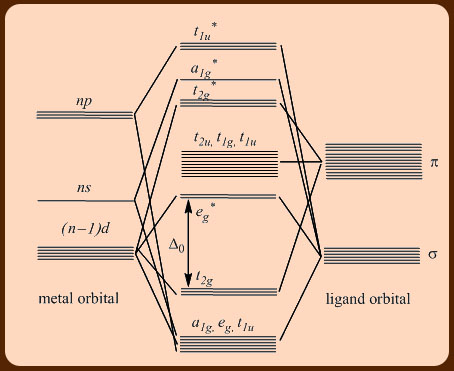
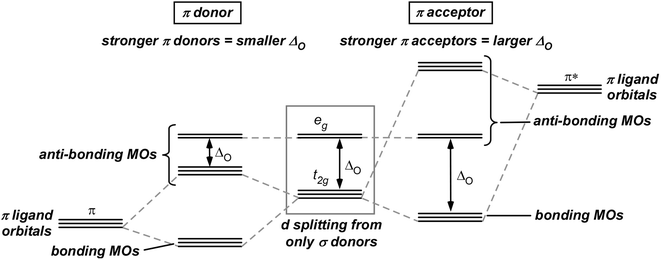
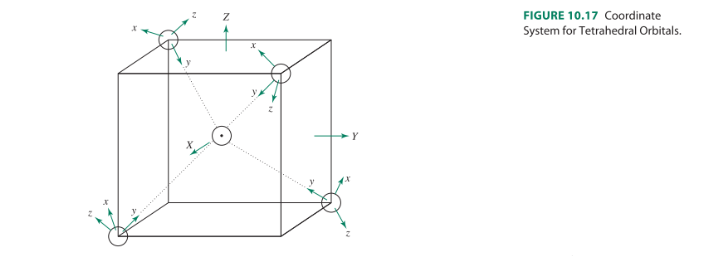
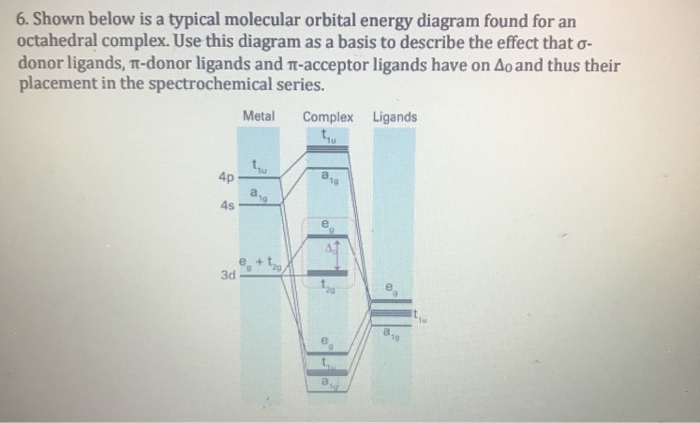



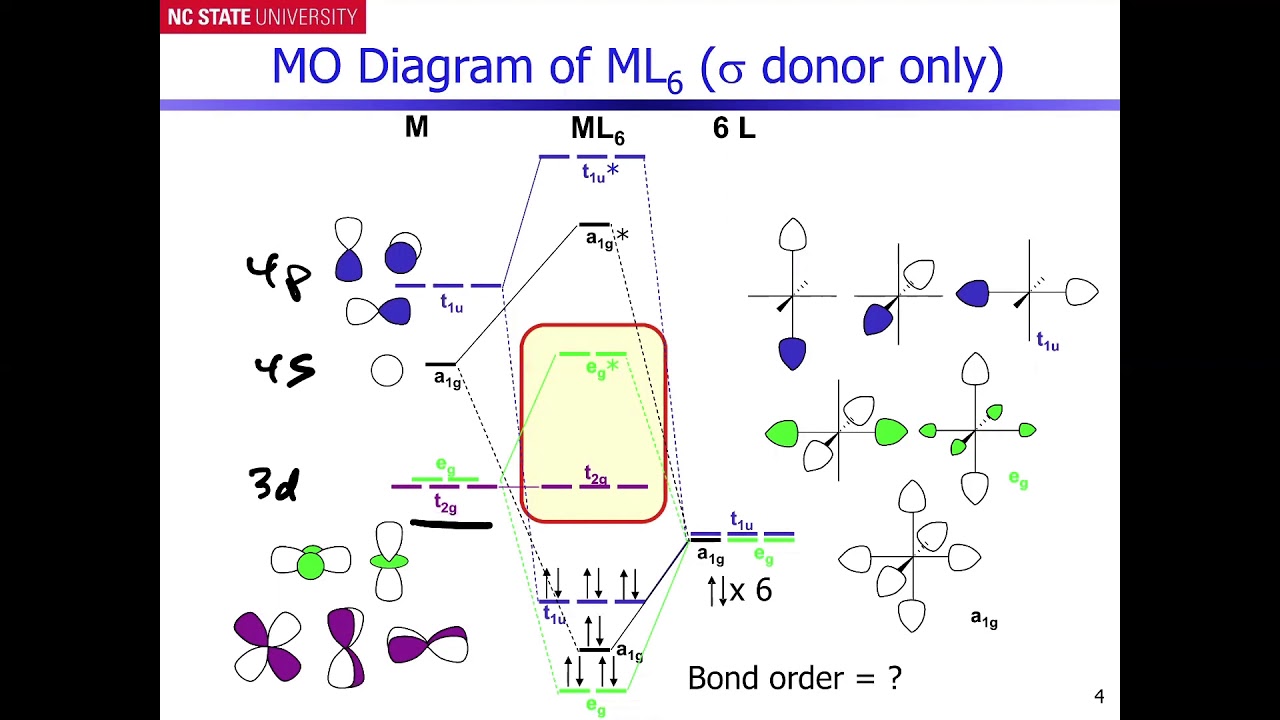
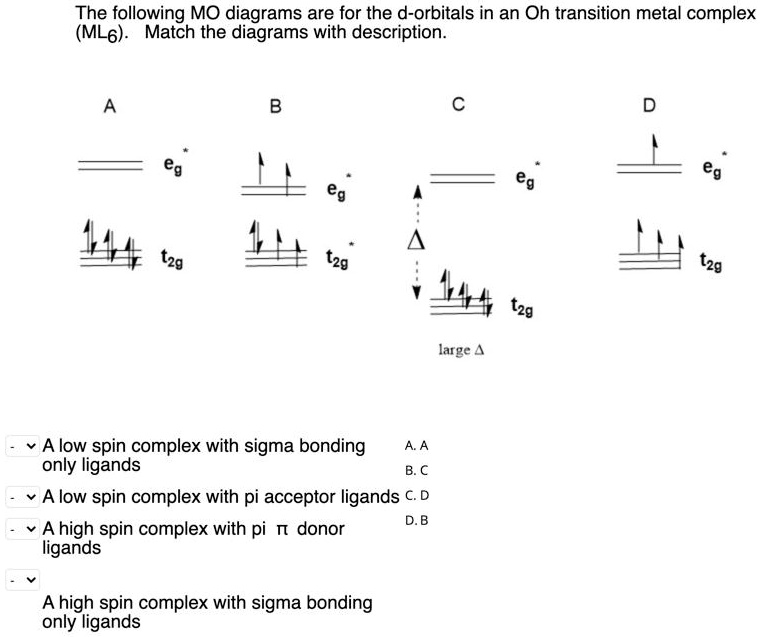
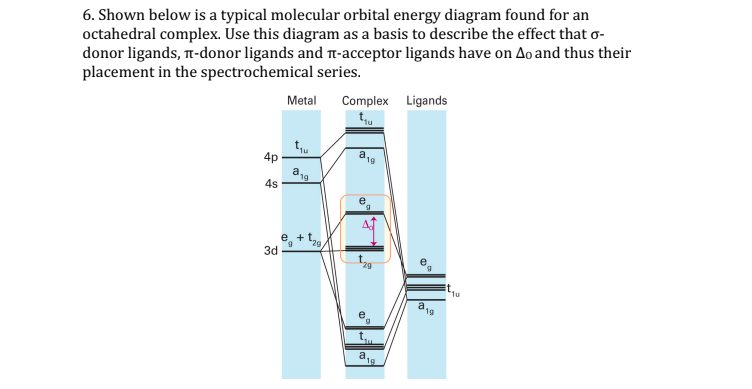
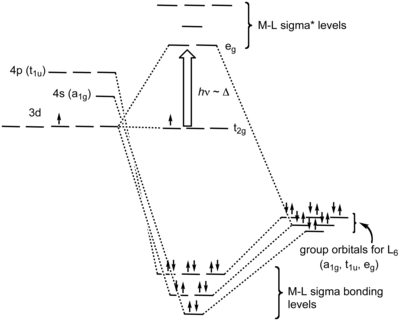





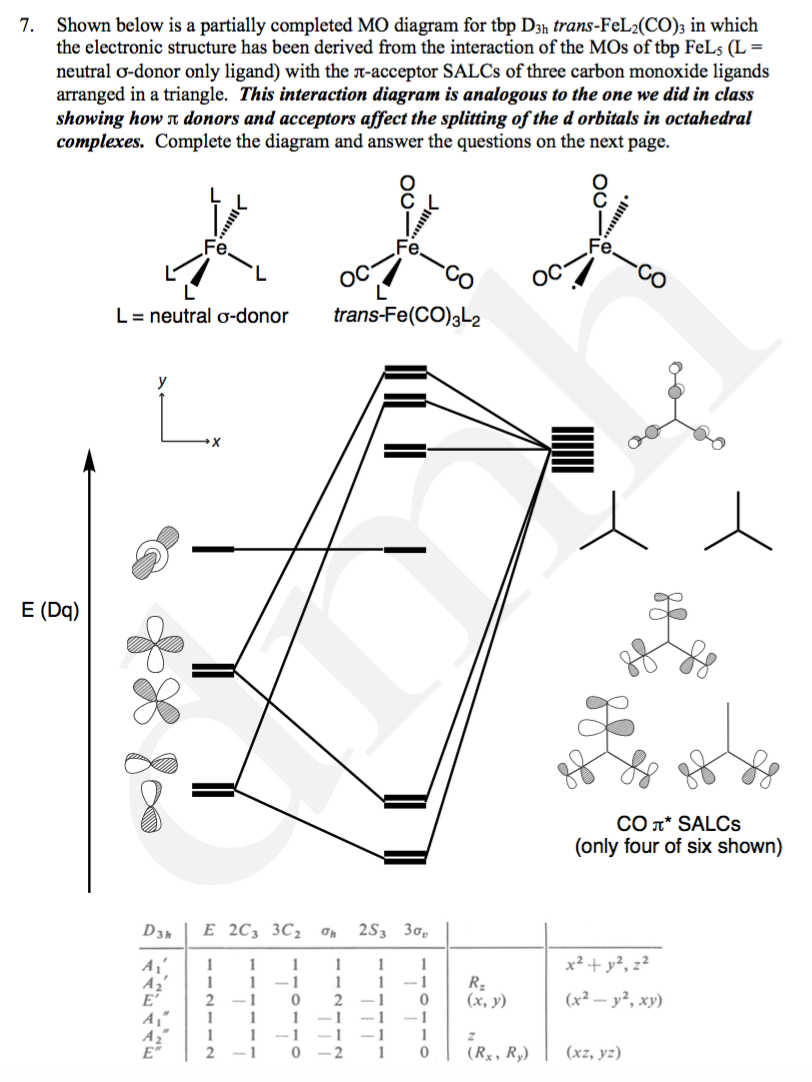



0 Response to "42 pi acceptor mo diagram"
Post a Comment