41 molecular orbital diagram for water
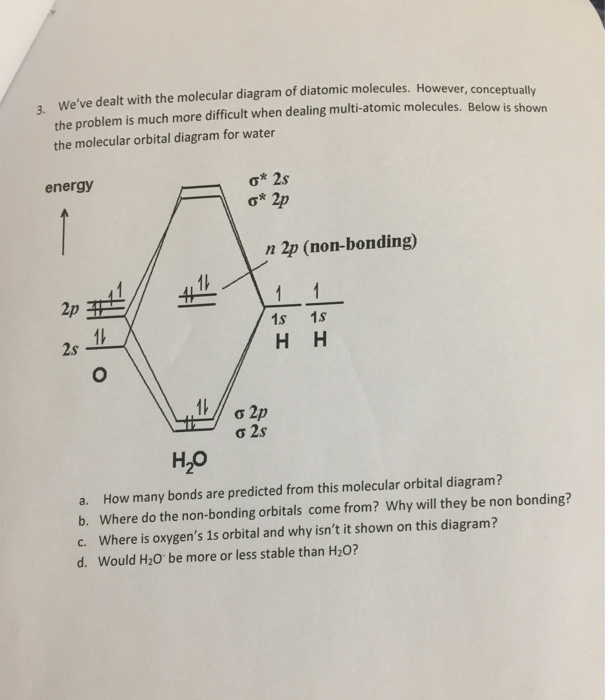
Solved Hybrid Orbitals: Complete the MO diagram of water ... Hybrid Orbitals: Complete the MO diagram of water - Copy. Part A. In this problem, you will build a hybrid molecular orbital diagram for water. First, place the valence atomic orbitals of hydrogen and oxygen, occupied with the correct numbers of electrons, in the correct columns (labeled "H atoms" and "O atom"). Molecular Orbital Theory - Chemistry Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution We draw a molecular orbital energy diagram similar to that shown in .
H2O Lewis Structure, Molecular Geometry, and Hybridization Molecular Orbital diagram of water (H2O) The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound. Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure.
Molecular orbital diagram for water
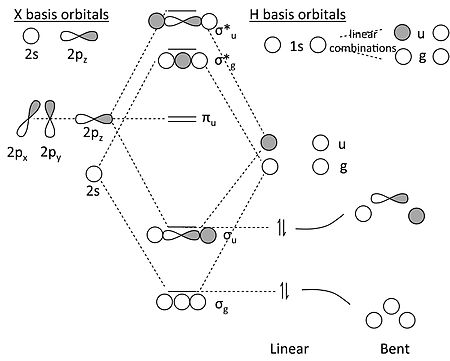
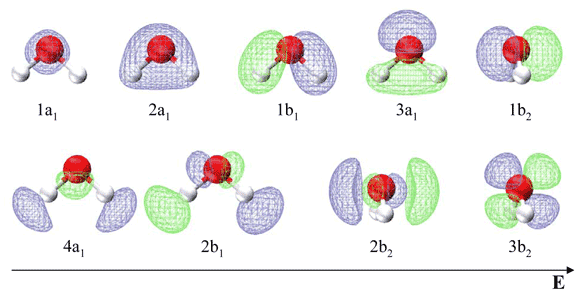
PDF Polyatomic Molecular Orbital Theory - La Salle University Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H Molecular orbitals for water (H2O) Molecular Orbitals for Water (H 2 O). The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2 (2a 1) 2 (1b 2) 2 (3a 1) 2 (1b 1) 2 (4a 1) 0 (2b 2) 0 (3b 2) 0 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. b (experimental data is given in []).They are set out with the lowest energy (that is, most ... Molecular orbital analysis of the hydrogen bonded water ... Figure 2: The orbital interaction diagram of (H2O)2. Orbital energy levels are represented as solid bars. The bars on the left and right sides correspond to the FOs of the two water monomers; the...
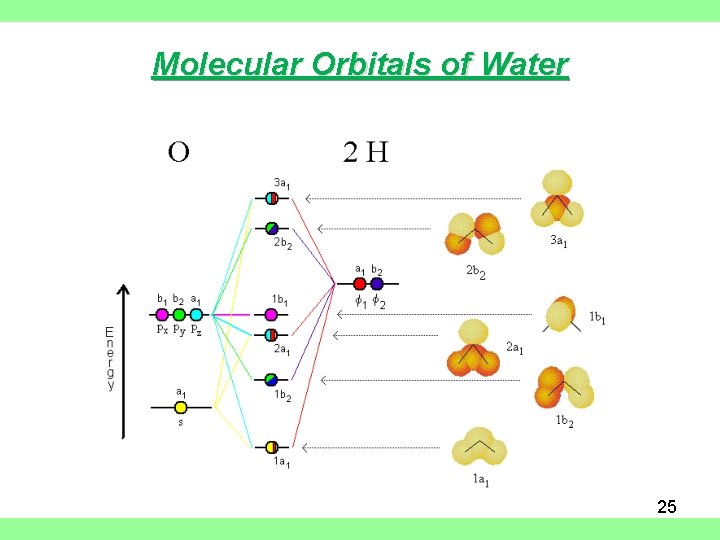
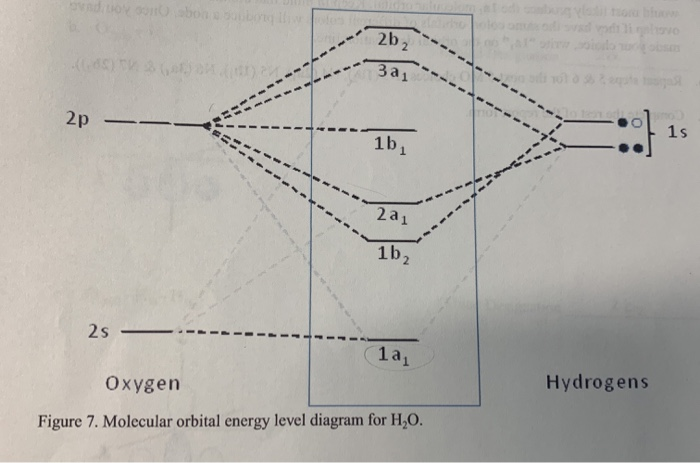
Molecular orbital diagram for water. Solved Report Sheet: Molecular Orbitals of Water 1. Match ... Water ( H 2 O) is an isolated molecule having five occupied and three unocc …. View the full answer. Transcribed image text: Report Sheet: Molecular Orbitals of Water 1. Match the orbital designations (1a, 2a, 3a, 1b, 1b, or 2b,) with each of the following possible combinations of atomic orbitals: a. 0+H +H. Orbital Designation b. 0:p+ H+H. H20 Molecular Orbital Diagram - schematron.org A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.Chemical bonding of H2O - WikipediaChemical bonding of H2O - Wikipedia Introduction to Molecular Orbital Theory In the water molecule the highest occupied orbital, (1b1) is non-bonding and highly localized on the oxygen atom, similar to the non-bonding orbitals of hydrogen fluoride. The next lowest orbital (2a1) can be thought of as a non-bonding orbital, as it has a lobe pointing away from the two hydrogens. PDF Molecular Orbitals for Water (H2O) - idc-online.com orbital in the gas phase is 539.9 eV [1227]. These orbitals are appreciably changed in ice and water; the experimental electron binding energies in liquid water being 2a 1 30.90 eV, 1b 2 17.34 eV, 3a 1 13.50 eV, 1b 1 11.16 eV [877]. The experimental binding energy of the 1a 1 orbital in the liquid phase consists of a broad energy distribution ...
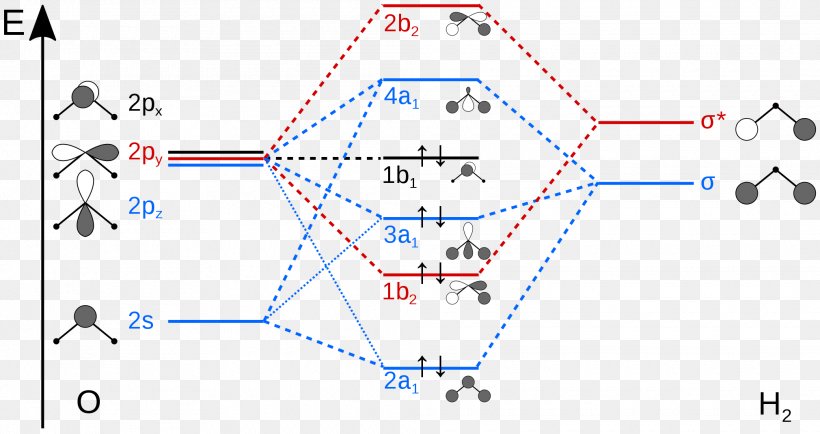
The MO Diagram for Water - Hunt Research Group The MO Diagram for Water. Revision. • molecular orbitals are combinations of atomic orbitals. • atomic orbitals: o atomic orbitals have a radial and angular.14 pages PDF Hybrid Molecular Orbitals - University of Illinois Urbana ... 6. There is one p orbital on boron but there is no adjacent atom with another p orbital. Add it to the molecular orbital diagram as a non-bonding molecular orbital. 7. There are a total of 6 electrons to add to the molecular orbital diagram, 3 from boron and 1 from each hydrogen atom. sp Hybrid Orbitals in BeH2 1. PDF Molecular Orbital Theory - Octahedral, Tetrahedral or ... molecular orbital. After the formation of molecular orbitals, both electrons occupy σ-orbital. Now, if the energy of σ-orbital is closer to ϕA, it will have more ϕA character and hence the electron density of both of the electrons will be concentrated more on atom A than B. Similarly if the energy of σ-orbital is closer to ϕB, it will have PDF Extended Pi Bonding - University of Illinois Urbana-Champaign In symmetry-based molecular orbital diagrams for the multiatom molecules water, ozone, and methane, we'll combine group orbitals with the valence orbitals of the central atom. The group orbitals are linear combinations of atomic orbitals from all the atoms bonded to the central atom. In the simpler bonding models, chemical bonds are regions
PDF Inorganic Chemistry with Doc M. - Creighton University 2. The 10-Step approach to making MO diagrams via symmetry considerations 3. Molecular orbital diagram for bent H2O 4. Molecular orbital diagram for linear BeH2 5. Molecular orbital diagram for square planer XeF4 6. Using p-orbitals for σ-bonding: molecular orbital diagram for trigonal planer BF3 1. H20 Molecular Orbital Diagram - Wiring Diagrams H20 Molecular Orbital Diagram. It uses 3-D pictorial presentations of molecular orbitals to elucidate organic reaction . As can be seen from the energy diagram - four of the molecular orbitals. General procedure for simple molecules that contain a central atom: build group orbitals using the outer atoms, then interact the group orbitals. Molecular Orbitals - Molecular Orbitals for Polyatomic ... In summary, the electronic configuration of the water molecule as determined by molecular orbital theory is 1a 21 2a 21 1b 22 3a 21 1b 21 The la 1 orbital is a nonbinding inner shell orbital. The pair of electrons in the la 1 orbital simply screen two of the nuclear charges on the oxygen from the protons. PDF MO Diagrams for Linear and Bent Molecules Water With the orbital shapes, symmetries, and energies in hand we can make the MO diagram! A1 A1 B1 B2 -15.8 eV -32.4 eV A1 B1 -13.6 eV 2a1 3a1 4a1 1b1 2b1 1b2 nb nb σ σ Two bonds, two lone pairs on O. HOMO is nonbonding.
Molecular Orbitals - Chem1 This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic orbitals.
Energy level diagram for the molecular orbitals of OH ). H ... The kinetics of the reactions of water, hydroxide ion and sulfide species with CO2, OCS and CS2 are investigated using the molecular orbital approach and available kinetic data.
PDF Some Considerations for Building Molecular Orbitals The water HOMO has B 1 symmetry The water HOMO is a pure oxygen 2p x orbital and does not have any contribution from H This lone-pair orbital is orthogonal to the molecular plane and is responsible for the basic/nucleophilic character of the water molecule 5.03 Inorganic Chemistry
PDF Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules 4 Lecture 2 Pi bond (π): bonding molecular orbital -The bonding electron density lies above and below, or in front and in back of the bonding axis, with no electron directly on the bonding axis, since 2p orbitals do not have any electron density at the nucleus.
EXP 6 Molecular Orbitals of Water .pdf - 6.1 Experiment 6 ... 6.3 Figure 5. A molecular orbital energy level diagram for dioxygen. Atomic p-orbitals may also overlap in a side-to-side manner. This type of overlap is referred to as pi (ð) overlap.These ð interactions are shown in Figure 4. In each of the previous three figures, note that the energy of the molecular bonding orbital is lower than the energy of the original atomic orbitals, while the ...
Molecular orbital diagram - Wikipedia Molecular orbital diagrams are diagrams of molecular orbital (MO) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (AO) energy levels for comparison, with the energy levels increasing from the bottom to the top. Lines, often dashed diagonal lines, connect MO levels with their constituent AO levels.
Molecular Orbital Theory - Purdue University Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
9.8: Molecular Orbital Theory - Chemistry LibreTexts Molecular Orbital Diagrams. This scheme of bonding and antibonding orbitals is usually depicted by a molecular orbital diagram such as the one shown here for the dihydrogen ion H 2 +. Atomic valence electrons (shown in boxes on the left and right) fill the lower-energy molecular orbitals before the higher ones, just as is the case for atomic ...
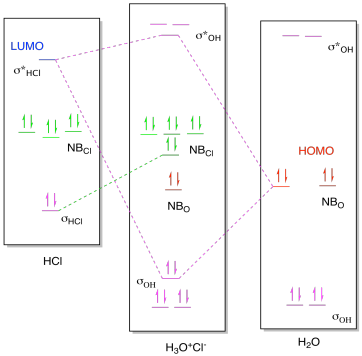
Chemical bonding of water - Wikipedia Hybridized Molecular Orbital (MO) diagram of H 2 O. To further distinguish the electron energy differences between the two non-bonding orbitals, orbital mixing can be further performed between the 2p (3a 1) orbital on oxygen and the antibonding 4a 1 orbital since they are of the same symmetry and close in energy level. Mixing these two orbitals ...
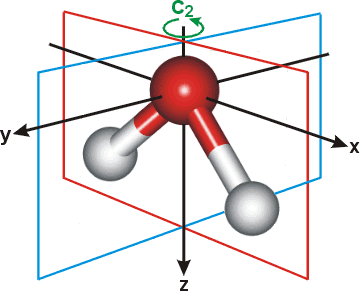
6.2.3: H2O - Chemistry LibreTexts We will walk through the steps below to construct the molecular orbital diagram of water. Preliminary Steps Step 1. Find the point group of the molecule and assign Cartesian coordinates so that z is the principle axis. The H 2 O molecule is bent and its point group is C 2 v. The z axis is collinear with principle axis, the C 2 axis.
Molecular Orbital (MO) Diagram of Polyatomic molecules ... In polyatomic molecules we can have more than two atoms combining, e.g. in case of beryllium hydride there are 3 atoms overlapping simultaneously. So in this...
Molecular orbital diagram for H2O (water) Molecule - YouTube About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
Molecular Orbital Theory - Detailed Explanation with ... The Molecular Orbital Theory (often abbreviated to MOT) is a theory on chemical bonding developed at the beginning of the twentieth century by F. Hund and R. S. Mulliken to describe the structure and properties of different molecules. The valence-bond theory failed to adequately explain how certain molecules contain two or more equivalent bonds ...
Molecular orbital analysis of the hydrogen bonded water ... Figure 2: The orbital interaction diagram of (H2O)2. Orbital energy levels are represented as solid bars. The bars on the left and right sides correspond to the FOs of the two water monomers; the...
Molecular orbitals for water (H2O) Molecular Orbitals for Water (H 2 O). The five occupied and the lowest three unoccupied molecular orbitals of the isolated molecule (1a 1) 2 (2a 1) 2 (1b 2) 2 (3a 1) 2 (1b 1) 2 (4a 1) 0 (2b 2) 0 (3b 2) 0 were calculated using the Restricted Hartree-Fock wave function (RHF) using the 6-31G** basis set. b (experimental data is given in []).They are set out with the lowest energy (that is, most ...
PDF Polyatomic Molecular Orbital Theory - La Salle University Molecular Orbital Theory - Walsh diagram The Walsh diagram shows what happens to the molecular orbitals for a set of molecules which are related in structure. In this case, the difference is the H-X-H bond angle which decreases from 180 o to 90 o Molecular Orbital Theory - Walsh diagram Water 104.5 ° X H H H O H
























0 Response to "41 molecular orbital diagram for water"
Post a Comment