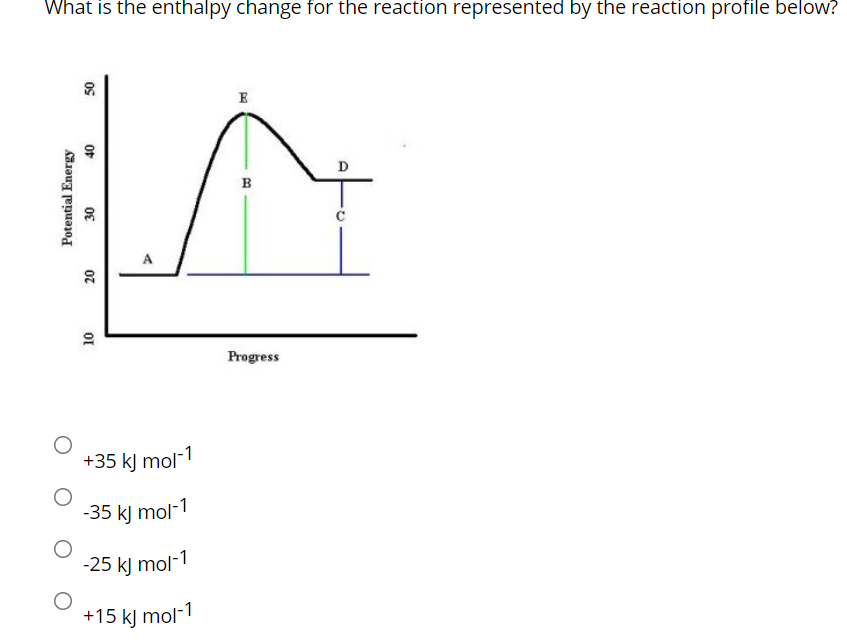
40 Which Of The Following Enthalpies Of Reaction Would The Reaction Represented By The Diagram Have
AP Chemistry: Midterm Review Flashcards - Quizlet The enthalpy of a reaction (ΔH) is estimated as the sum of the bond enthalpies of the bonds broken minus the sum of the bond enthalpies of the bonds formed. No bonds are formed during the dissociation reaction, so ΔH is equal to the energy required to break the O=O double bond, which is 495kJ/mol. BITSAT 16 Years Chapter-wise Solved Papers (2020 - 2005) ... Which of the following diagram represents the same process? [2020] B A P C D V D C V 10. In the reaction 5 4 6 2PCl U PCl+ + PCl- , the change in ...
Thermochemistry Practice Test 2020 Flashcards - Quizlet The enthalpy change for the reaction represented above is ΔHT. This reaction can be broken down into a series of steps as shown in the diagram: A relationship that must exist among the various enthalpy changes is ΔHT - ΔH1 - ΔH2 - ΔH3 = 0 2 NH3(g) → 3 H2(g) + N2(g) ΔH°298 = 92 kJ/molrxn
Which of the following enthalpies of reaction would the reaction represented by the diagram have
PDF Enthalpy Changes - WordPress.com surroundings as a result of reactions, in accordance with the law of conservation of energy. The enthalpy change of reaction, ΔH, is the enthalpy change when the number of moles of reactants, as specified in a balanced equation, react together. An exothermic reaction is a reaction in which energy is transferred from the chemical Answer: 2C2H2 + 5 O2 → 4CO2 + 2H2O ... - Clutch Prep Example: The reaction of methane with chlorine gas is illustrated by the reaction below:Calculate the ∆Horxn if the standard enthalpies of formation for CH4 , CCl4 , and HCl are -74.87 kJ/mol, -139 kJ/mol and -92.31 kJ/mol respectively. apcentral.collegeboard.org › pdf › ap-chemistry-frqAP Chemistry 2017 Free-Response Questions - AP Central (b) At 30°C the reaction is thermodynamically favorable, but no reaction is observed to occur. However, at 120°C, the reaction occurs at an observable rate. (i) Explain how the higher temperature affects the collisions between the reactant molecules so that the reaction occurs at an observable rate at 120°C.
Which of the following enthalpies of reaction would the reaction represented by the diagram have. The Energy in Chemical Reactions: Thermodynamics and ... As we have seen, if a chemist has a thermometer and can carefully perform a controlled chemical reaction, the result is a direct measure of the heat that this reaction involved. If this reaction was carried out under constant pressure, of which our planet is a good example, then this heat is really a change in enthalpy. In the end, chemists are looking for the change in enthalpy for a reaction. Calculate the enthalpy change of formation for the ... For the reaction shown below complete the following calculations. H2(g) + C2H4(g) --> C2H6(g) (a) Estimate the enthalpy of reaction using the bond energy values in Table 9.4. (b) Calculate the enthalpy of reaction, using standard enthalpies of formation. Chem. Change in enthalpy problem. Consider the reaction represented below. A scientist measures the standard enthalpy change ... - Jiskha A scientist measures the standard enthalpy change for the following reaction to be -2923.0 kJ : 2C2H6(g) + 7 O2(g) 4CO2(g) + 6 H2O(g) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation . Chem. Change in enthalpy problem. Consider the reaction represented below. PDF 3.2.1. Enthalpy changes - chemrevise In any reaction where the reactants are dissolved in water we assume that the heat capacity is the same as pure water. Also assume that the solutions have the density of water, which is 1g cm-3. Eg 25cm3 will weigh 25 g Example 1. Calculate the enthalpy change of reaction for the reaction where 25cm3 of 0.2 M
Hess's Law and enthalpy change calculations - chemguide If you go via the intermediates, you do have to put in some extra heat energy to start with, but you get it back again in the second stage of the reaction sequence. However many stages the reaction is done in, ultimately the overall enthalpy change will be the same, because the positions of the reactants and products on an enthalpy diagram will ... Chapter 5, Thermochemistry Video Solutions ... - Numerade Problem 78. The complete combustion of 1 mol of C 4 H 10 O ( l) to C O 2 ( g) and H 2 O ( l) yields Δ H ∘ = − 2723.7 k J . (a) Write a balanced equation for the combustion of 1 m o l of C 4 H 10 O ( l). (b) By using the information in this problem and data in Table 5.3, calculate Δ H f ∘ for diethyl ether. 5.4: Enthalpy of Reaction - Chemistry LibreTexts To measure the energy changes that occur in chemical reactions, chemists usually use a related thermodynamic quantity called enthalpy ( H) (from the Greek enthalpein, meaning "to warm"). The enthalpy of a system is defined as the sum of its internal energy U plus the product of its pressure P and volume V: (5.4.2) H = U + P V. 7.4: Standard Enthalpy of Formation - Chemistry LibreTexts Example 7.4.1. Given a simple chemical equation with the variables A, B and C representing different compounds: A + B ⇋ C. and the standard enthalpy of formation values: ΔH fo [A] = 433 KJ/mol. ΔH fo [B] = -256 KJ/mol. ΔH fo [C] = 523 KJ/mol. the equation for the standard enthalpy change of formation is as follows:
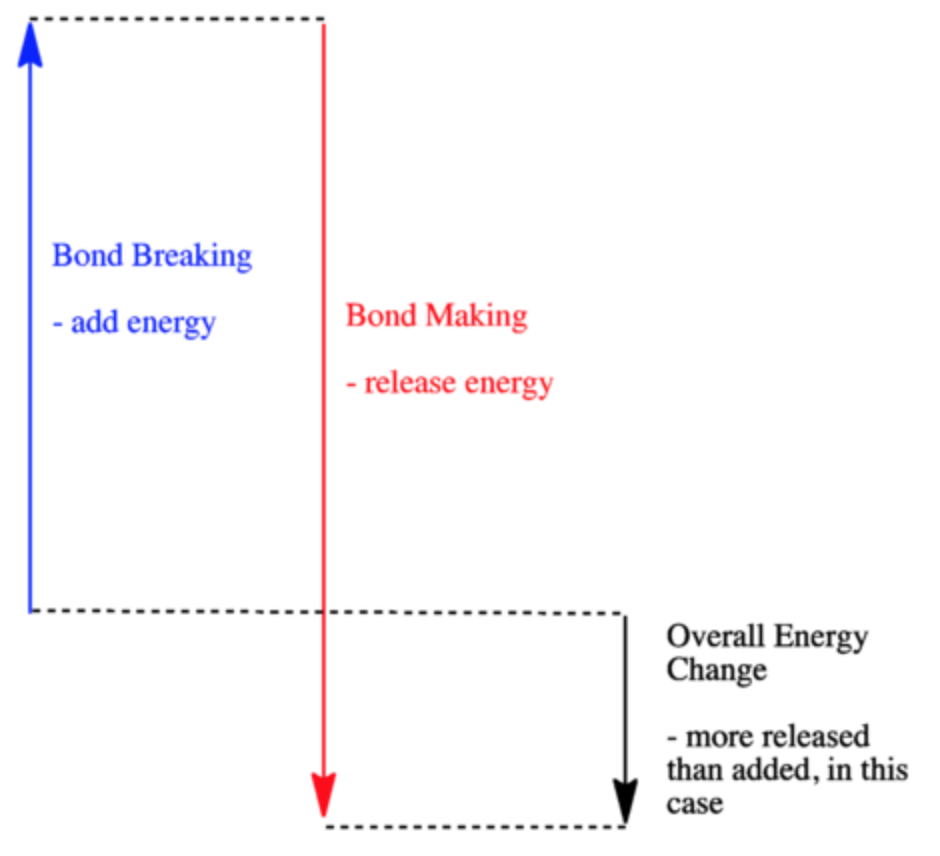
AP Chemistry Unit 5 Progress Check Flashcards - Quizlet The overall reaction represented above is proposed to take place through two elementary steps. Which of the following statements about the chemical equation for step 1 and the rate law for the overall reaction is correct? The chemical equation for step 1 is NO2(g)+F2(g)→NO2F(g)+F(g), and the rate law for the overall reaction is rate=k[NO2][F2]. … 8.3: Enthalpy and Hess' Law - Chemistry LibreTexts In a thermochemical equation, the enthalpy change of a reaction is shown as a Δ H value following the equation for the reaction. This ΔH value indicates the amount of heat associated with the reaction involving the number of moles of reactants and products as shown in the chemical equation. Covalent Bond: Types of Bonds, Examples, Formation - Embibe 17.01.2022 · Covalent bonding in molecular substances is represented by the Lewis electron dot diagram. For example, the Lewis diagram of two hydrogen atoms is: When two hydrogen atoms share electrons, the lewis diagram is as shown below: This depiction of molecules sharing electrons is shown by using a dash. This dash represents a covalent bond. The hydrogen … energetics MC quiz Flashcards | Quizlet A. Bond enthalpies have positive values for strong bonds and negative values for weak bonds. B. Bond enthalpy values are greater for ionic bonds than for covalent bonds. C. Bond breaking is endothermic and bond making is exothermic. D. The carbon-carbon bond enthalpy values are the same in ethane and ethene. c.
› 36546170 › Vollhardt_Organic(PDF) Vollhardt Organic Chemistry Structure Function 6th ... Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF. Download. Vollhardt Organic Chemistry Structure Function 6th txtbk.PDF
DOCUMENTATION OF DISTILLATION COLUMN DESIGN - … Academia.edu is a platform for academics to share research papers.
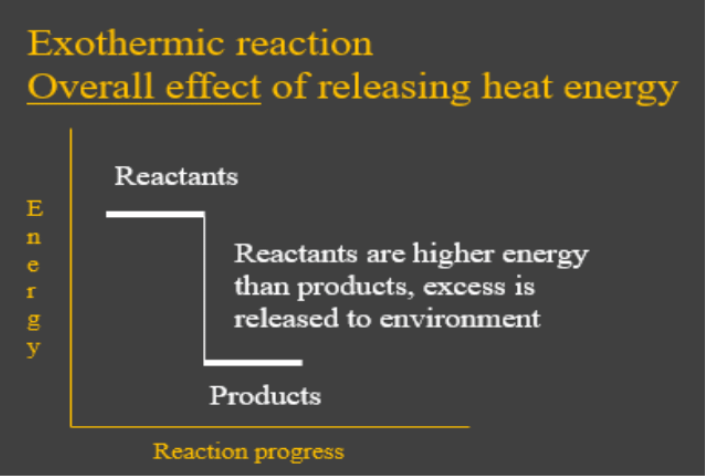
Chemistry 4.09 Quiz: Writing Thermochemical Equations ... Which of the following enthalpies of reaction would the reaction represented by the diagram have? ΔH < 0. Related questions. QUESTION. when 878 J of heat are absorbed by 125 g of silver, the temperature rises from 25.0 ºC to 54.9 ºC. what is the specific heat capacity of silver?
Answered: 2C(s) + 2H2O(g)→ CH4(g) + CO2(g)… | bartleby Science Chemistry Q&A Library 2C (s) + 2H2O (g)→ CH4 (g) + CO2 (g) Determine AH for this reaction using the following enthalpies of reactions: C (s) + H2O (g) → CO (g)+ H2 (g) CO (g) + H2O (g) → CO2 (g) + H2 (g) CH4 (g) + H2O (g) → 3H2 (g) + CO (g) AH = 131.3 kJ AH = - 41.2 kJ AH = 206.1 kJ.
Essential AS Chemistry for OCR - Page 127 - Google Books Result Ted Lister, Janet Renshaw · 2004 · ChemistryIn an endothermic reaction the products end up with more energy than the starting materials , so AH is positive . 13.2.2 Enthalpy profile diagrams We use ...
CHM1045 Enthalpy Lecture - Department of Chemistry ... Standard Enthalpy of Reaction. The standard enthalpy of reaction (ΔH o Rxn) is the enthalpy of a reaction carried out at 1 atm. We have already learned one process by which we can calculate the Enthalpy of Reaction in Calorimetry. There are two other methods we will learn now: 1) Heat of Reaction from Standard Heats of Formation
› userfiles › 1286Topic 5.1 Exothermic and Endothermic Reactions Heat and ... In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic.
36 what is the enthalpy of reaction from the following ... Energy diagrams for these processes will often plot the enthalpy (H) instead of Free Energy for simplicity. Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change). If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings.
PDF 6 g What is the standard enthalphy change ΔH Which of the following expressions is equivalent to ΔHo for the reaction represented above? A x + y B x - y C x + 2y D 5. How much heat is released or absorbed when 0.050 mol of Cl2(g) is formed from KCl(s)? 87.4 kJ is released 43.7 kJ is released 43.7 kJ is absorbed 87.4 kJ is absorbed 6.
en.wikipedia.org › wiki › Standard_enthalpy_of_formationStandard enthalpy of formation - Wikipedia For example, the standard enthalpy of formation of carbon dioxide would be the enthalpy of the following reaction under the above conditions: C(s, graphite) + O 2 (g) → CO 2 (g) All elements are written in their standard states, and one mole of product is formed. This is true for all enthalpies of formation.
ChemTeam: Hess' Law - using standard enthalpies of formation All the above values have units of kJ/mol because these are standard values. All standard enthalpies have the unit kJ/mol. As a brief reminder, here is the chemical reaction for the standard enthalpy of glucose: 6C(s, graphite) + 6H 2 (g) + 3O 2 (g) ---> C 6 H 12 O 6 (s) Each standard enthalpy value is associated with a chemical reaction.
OAT 2017-2018 Strategies, Practice & Review with 2 Practice ... Kaplan Test Prep · 2016 · MedicalNot all collisions, however, result in a chemical reaction. ... which the reaction has progressed from reactants to products; see below) can be represented ...
Assisting students with assignments online - Success Essays We cover any subject you have. Set the deadline and keep calm. Receive your papers on time. Detailed Writer Profiles. That’s our Place of Truth. Look over the writers’ ratings, success rating, and the feedback left by other students. Email and SMS Notifications. Stay informed 24⁄7 about every update of the whole ordering process. Plagiarism Free Papers. We double-check all the ...
Heat of Neutralization: HCl(aq) + NaOH(aq) | Chemdemos HCl(aq) + NaOH(aq) --> NaCl(aq) + H 2 O(l) + Energy. Thermochemistry determine the heat exchanged at constant pressure, q = m c ∆T.. Calculating the limiting reactant, the change in enthalpy of the reaction, ∆H rxn, can be determined since the reaction was conducted under conditions of constant pressure ∆H rxn = q rxn / # moles of limiting reactant. This reaction is classified as an ...
Enthalpy of Chemical Reactions - Nassau Community College reaction. Consider the following general type of reaction . reactant Æ products . The ∆H is defined as the difference between the enthalpies of products and the reactants. Thus . ∆H = Hproducts - Hreactants . The enthalpy of reaction can be positive or negative or zero depending upon whether the heat is gained or lost or no heat is lost or ...
(PDF) Chemistry - Gilbert | Tín Phạm - Academia.edu Academia.edu is a platform for academics to share research papers.
How would you calculate the standard enthalpy change for ... DeltaH_"rxn" = +"365 kJ" Start by writing down the reaction 2"A" + "B" -> 2"C" + 2"D" Now, I assume that the values given to you represent the respective standard enthalpies of formation for the species that take part in the reaction. Standard enthalpies of formation are measured when one mole of each of these compounds is formed from its constituent elements in their pure state.
AP CHEM FINAL Flashcards | Quizlet A stoichiometric mixture of CO(g) and H2(g) was allowed to react in two different 2.0L rigid containers at a constant temperature of 298K. The reaction is represented by the equation above. Diagram 1 represents the uncatalyzed reaction and diagram 2 represents the catalyzed reaction one hour after the reactants were mixed.
which of the following enthalpies of reaction would the ... The x-axis represents the reaction progress. Chemical reactions proceed (or are read) from left to right. Therefore, looking at the potential energy diagram, ...1 answer · 3 votes: In chemical reactions, the internal energy represents the total energy of the system and is often called Enthalpy. The x-axis represents the reaction progress. ...
Chapter 6 thermodynamics class 11 cbse - SlideShare 02.07.2015 · ∆H measurements We can measure heat change at constant pressure in a calorimeter by the following technique:- We know that ∆H = qp and therefore heat absorbed or evolved qp . At constant pressure is also called heat of reaction or enthalpy of reaction(∆rH).In an exothermic reaction, heat is evolved and system loses heat to the surroundings . Therefore …
Chemistry: The Molecular Nature of Matter Neil D. Jespersen, Alison Hyslop · 2021 · ScienceTOOLS Enthalpy diagram The energy relationships among the alternative pathways for the same overall reaction can be depicted using a graphical construction ...
Calculate the enthalpy of the reaction 2B2H6 + 6O2=2B2O3 ... For the reaction shown below complete the following calculations. H2(g) + C2H4(g) --> C2H6(g) (a) Estimate the enthalpy of reaction using the bond energy values in Table 9.4. (b) Calculate the enthalpy of reaction, using standard enthalpies of formation. chemistry. The reaction of iron with oxygen is very familiar.
5.7: Enthalpy Calculations - Chemistry LibreTexts Figure \(\PageIndex{3}\): Process diagram showing the general process for using enthalpies of formation to calculate enthalpies of reaction. In essence we break apart the reactants into their atoms at standard state (the opposite of formation) in the first step, and then form the products from those atoms in the second step.
PDF CHAPTER 5: ANSWERS TO ASSIGNED PROBLEMS Hauser- General ... 5.69 The following is known as the thermite reaction 2 Al (s) + Fe 2O 3 (s) Al 2O 3 (s) + 2 Fe (s) This highly exothermic reaction is used for welding massive units, such as propellers for large ships. Using standard enthalpies of formation in Appendix C, calculate ΔH° for this reaction. Use Enthalpy of Formation data and equation:
› science › articleThermal runaway mechanism of lithium ion ... - ScienceDirect Jan 01, 2018 · The reaction at the anode at the third stage is less complex than the balanced reaction at the second stage. Besides the calorimetric results in [76] , [77] , [78] , Chen et al. [89] also confirmed that the reaction peak of the third stage stands for the reaction at the anode, by testing several kinds of graphite anode.
Which of the following enthalpies of reaction would the reaction ... Correct ✓ answer ✓ - Which of the following enthalpies of reaction would the reaction represented by the diagram have?3 answers · 3 votes: In chemical reactions, the internal energy represents the total energy of the system and is often ca...
CH 110_ Chapter 5 & 6 HW problems.pdf - Name John Marrero ... What is ∆H for the reaction represented by this equation?-∆H = 1453 kJ ... Calculate the enthalpy change for the reaction P 4 O 6 (s) + 2 O 2 (g) → P 4 O 10 (s) given the following enthalpies of reaction: P 4 (s) + 3 O 2 ... -Each box in an orbital diagram represents an orbital. (d) What object is represented by the half arrows in an ...
chem.libretexts.org › Bookshelves › AnalyticalStandard Potentials - Chemistry LibreTexts The overall redox reaction is composed of a reduction half-reaction and an oxidation half-reaction. From the standard electrode potentials listed Table P1 , we find the corresponding half-reactions that describe the reduction of H + ions in water to H 2 and the oxidation of Al to Al 3 + in basic solution:
apcentral.collegeboard.org › pdf › ap-chemistry-frqAP Chemistry 2017 Free-Response Questions - AP Central (b) At 30°C the reaction is thermodynamically favorable, but no reaction is observed to occur. However, at 120°C, the reaction occurs at an observable rate. (i) Explain how the higher temperature affects the collisions between the reactant molecules so that the reaction occurs at an observable rate at 120°C.
Answer: 2C2H2 + 5 O2 → 4CO2 + 2H2O ... - Clutch Prep Example: The reaction of methane with chlorine gas is illustrated by the reaction below:Calculate the ∆Horxn if the standard enthalpies of formation for CH4 , CCl4 , and HCl are -74.87 kJ/mol, -139 kJ/mol and -92.31 kJ/mol respectively.
PDF Enthalpy Changes - WordPress.com surroundings as a result of reactions, in accordance with the law of conservation of energy. The enthalpy change of reaction, ΔH, is the enthalpy change when the number of moles of reactants, as specified in a balanced equation, react together. An exothermic reaction is a reaction in which energy is transferred from the chemical






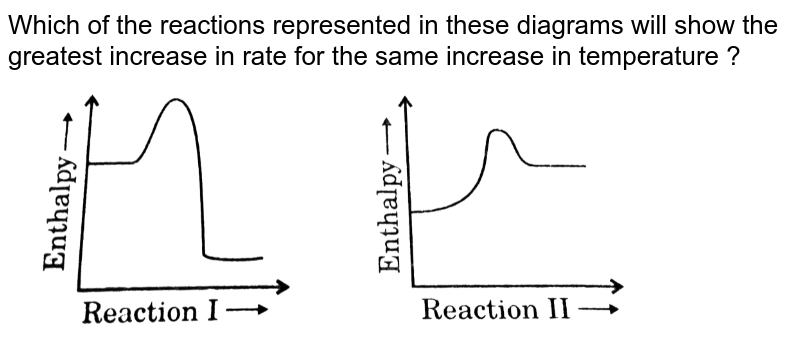



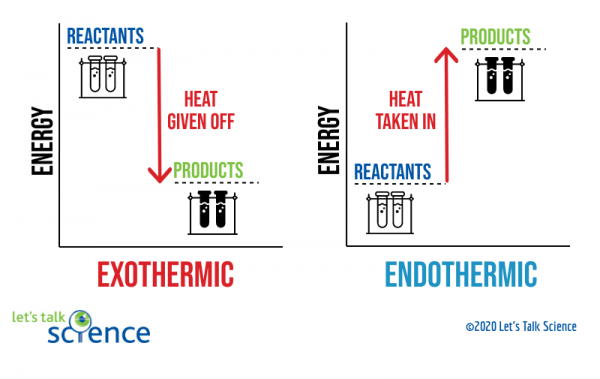

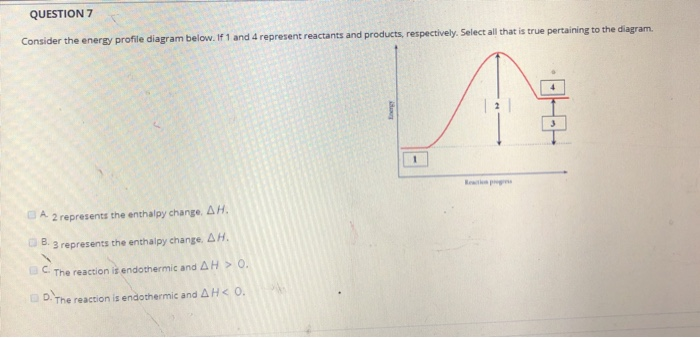

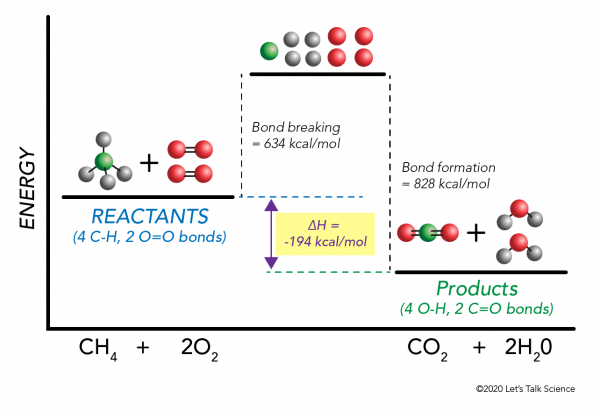




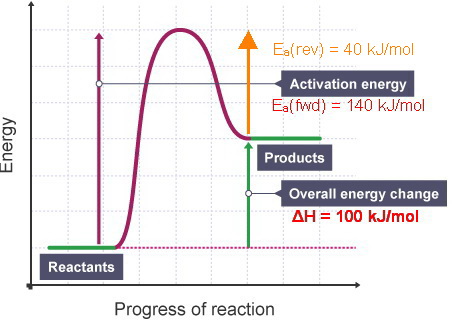
0 Response to "40 Which Of The Following Enthalpies Of Reaction Would The Reaction Represented By The Diagram Have"
Post a Comment