44 lewis dot diagram for no3
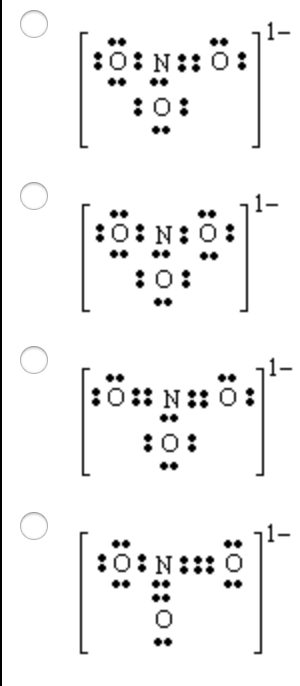
1-A molecular model of NO3- is shown below. Based on the best Lewis electron-dot structure for NO3- and formal charge considerations, what is the predicted N-O bond order for each N-O bond? 2 -How many resonance structures are there for SO3? Best Answer. This is the best answer based on feedback and ratings. Answer : The correct Lewis-dot structure of nitrate ion is shown below and the nitrogen has 4 covalent bonds. Lewis-dot structure : It shows the bonding between the atoms of a molecule and it also shows the unpaired electrons present in the molecule. The number of valance electrons are shown by 'dot'.
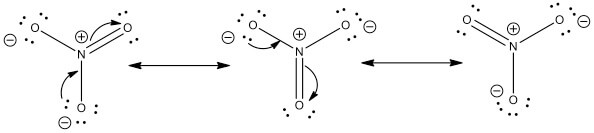
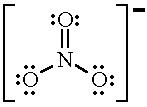
In lewis structure of NO 3- ion, there are three lone pairs (in the last shell) in two oxygen atom and that oxygen atoms. Also, those two oxygen atoms has a -1 charge. There is another oxygen atom. That oxygen atom is connected to the nitrogen atom by a double bond has two lone pairs in its last shell. Also, there is no charge in that oxygen atom.
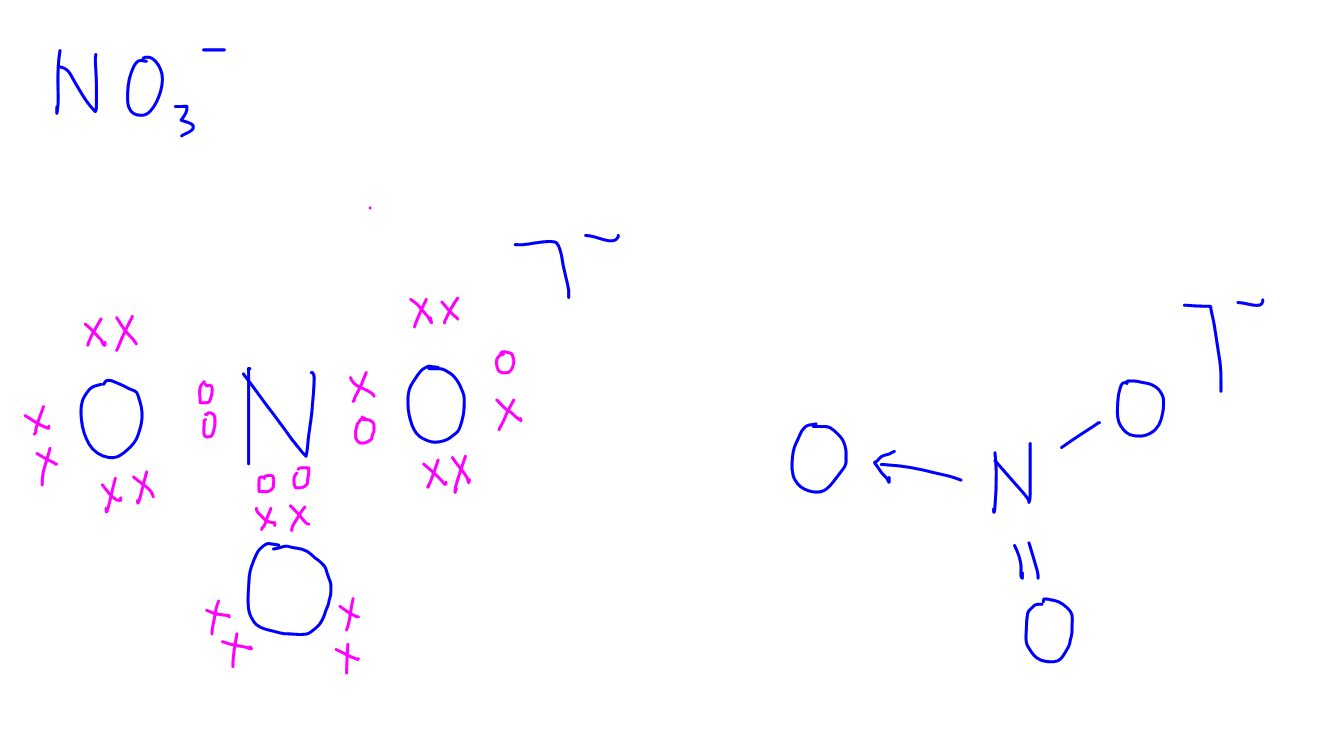
Lewis dot diagram for no3
Lewis Dot of the Nitrate Ion. NO 3-. Back. 70 More Lewis Dot Structures. The nitrate ion cannot be satisfactorily represented by just one Lewis Dots structure. All the bonds are the same length and must be thought of as a hybrid of multiple resonance structures. YouTube. Science Chemistry Q&A Library a) Draw one complete Lewis dot structure for the nitrate ion (NO3 - ) b) Determine the formal charge on each atom in the structure. a) Draw one complete Lewis dot structure for the nitrate ion (NO3 - ) b) Determine the formal charge on each atom in the structure. Problem Details. There are 3 Lewis structures for [ NO 3] - draw all three and compare polarity of the 3. Q. Draw the Lewis dot structure for CH2Cl2. Is this molecule polar?
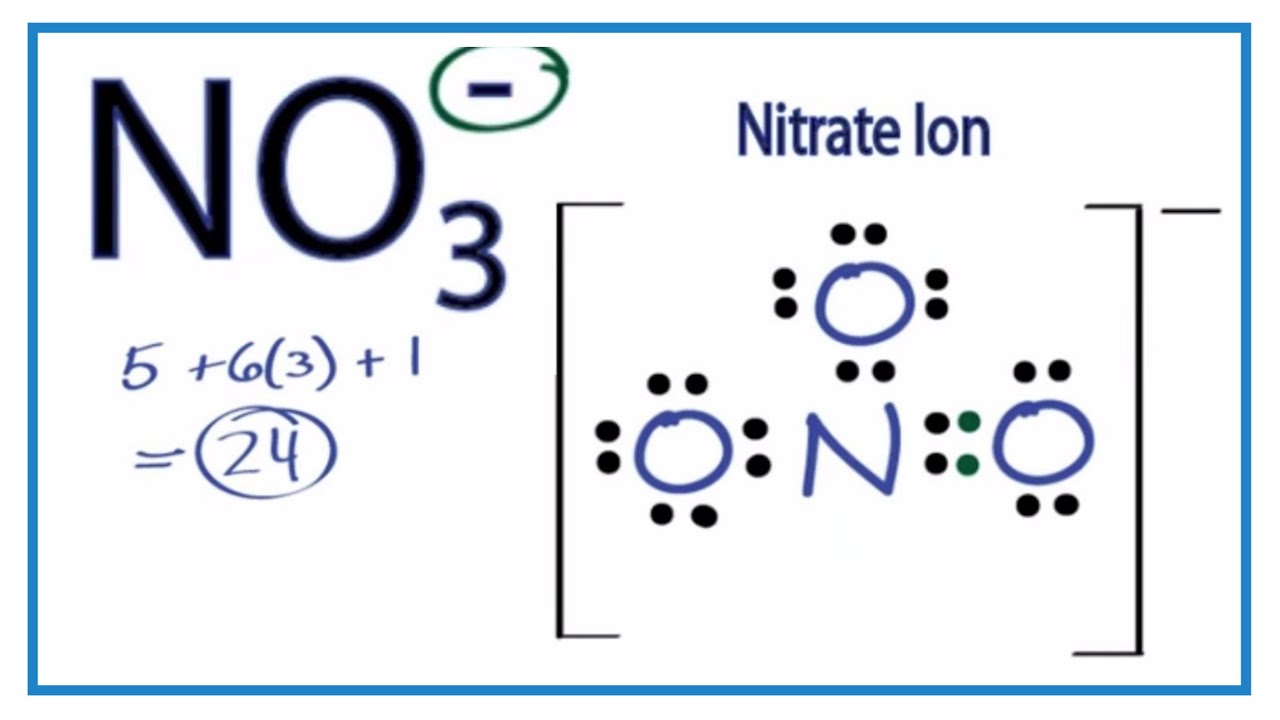
Lewis dot diagram for no3. In the Lewis structure of NO3- there are a total of 24 valence electrons. For the Lewis structure for NO3- you should take formal charges into account to find the best Lewis structure for the molecule. It will be necessary to create a double bond between an Oxygen (O) and a Nitrogen (N) atom for the Lewis structure to work. What is the lewis structure for NO3 -1? The Lewis structure begins with an N atom in the center with two O atoms singly bonded. Each of these O atoms has three pair of dots on the unbonded sides.... Problem 35 Easy Difficulty. Draw a Lewis dot diagram for $\mathrm{NO}_{3}^{-}$, and use the oxidation-state method of electron bookkeeping to determine how many electrons each atom should be assigned. Lewis electron-dot diagrams for CO2 and SO2 are given above. The molecular geometry and polarity of the two substances are A. the same because the molecular formulas are similar B. the same because C and S have similar electronegativity values C. different because the lone pair of electrons on the S atom make it the negative end of a dipole
Chemistry Q&A Library Draw a lewis dot structure for Agcl and Co(NO3)2. Draw a lewis dot structure for Agcl and Co(NO3)2. close. Start your trial now! First week only $4.99! arrow_forward. Question. Draw a lewis dot structure for Agcl and Co(NO3)2. check_circle Expert Answer. Want to see the step-by-step answer? Drawing the Lewis Structure for NO 3- (Nitrate Ion) Viewing Notes: The Lewis structure for NO 3- (Nitrate Ion) comes up quite often in chemistry. Be sure to put brackets, along with a negative sign, around the NO 3- Lewis structure when you are done to show that it is an ion with a negative charge. NO 3- has a total of 24 valence electrons. A step-by-step explanation of how to draw the NO3- Lewis Structure (Nitrate Ion). Get more chemistry help at www.Breslyn.org.For the NO3- Lewis structure,... Pembuatan Senyawa kompleks asetial asetanoat. Enter the email address you signed up with and we'll email you a reset link.
What is the lewis structure for NO3 -1? The Lewis structure begins with an N atom in the center with two O atoms singly bonded. Each of these O atoms has three pair of dots on the unbonded sides.... Unformatted text preview: How to draw Lewis Diagrams An outline of how to detemine the "best" Lewis structure for an example, NO3- is given below: 1.Determine the total number of valence electrons in a molecule 2. Draw a skeleton for the molecule which connects all atoms using only single bonds. Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond. I quickly take you through how to draw the Lewis Structure of NO3- (Nitrate Ion). I also go over the resonance, hybridization, shape and bond angle.
For the NO3- Lewis structure, calculate the total number of valence electrons for the NO3- molecule. After determining how many valence electrons there are in NO2, place them around the central atom to complete the octets. In the Lewis structure of NO3- there are a total of 24 valence electrons.
Given the ONE resonance isomer, there are 8 (unshared) lone pairs on the oxygen atoms. (O=)N^(+)(-O)_2^(-) is the typical resonance isomer. Such a structure demands that the doubly bound oxygen has 2 lone pairs, and each of the singly bound oxygen have 3 lone pairs. Because there are 7 valence electrons around each singly bound oxygen atom, these atoms have a formal negative charge.
Construction of NO3 Lewis Dot Structure. 1. In the ion NO3, there is 1 atom of nitrogen and 3 atoms of oxygen. It also has one negative charge. 2. Nitrogen and oxygen belong to periods 5A and 6A groups respectively in the periodic table. Hence, oxygen has 6 and nitrogen has 5 valence electrons in their outer shell. 3.
Lewis Electron-Dot Structure for NO3. Chemistry. Instructional. An Introduction to Chemistry. General Chemistry Modules. General Chemistry Problems.
Chemistry questions and answers. Consider the nitrate ion (NO3-). After drawing the correct Lewis dot structure (s), you would see: two possible resonance structures. two single bonds and one double bond around the nitrogen atom four single bonds around the central nitrogen atom. two double bonds around the central nitrogen atom.
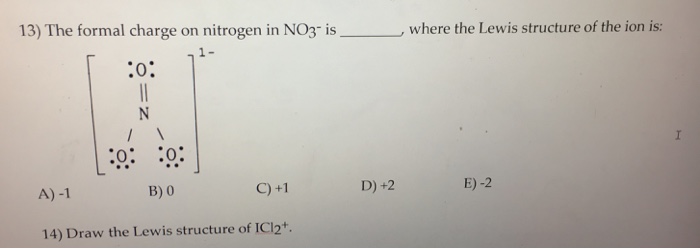
The Lewis representation of NO3 is aligned in a way where the nitrogen atom is in the center and orbited by three oxygen atoms. The nitrogen has a positive charge because it has 4 bonding electrons - 2 from the oxygen double bond and 1 from each of the N - O bonds.
Well, you gots a potassium cation, and nitrate anion as the gegenion.. Nitrate anion is an interesting customer in terms of resonance, in that THREE of the FOUR atoms bear a formal electric charge in the resonance structure. And of course the charges SUM to the formal charge of nitrate anion, i.e. negative ONE... And so for NO_3^(-)...we add up the valence electrons, i.e. 5_"nitrogen electrons ...
The formula for lithium oxide is Li 2 O. Jul 26, 2017 · Lewis structures or electron dot diagrams for atoms ions ionic compounds and covalent compounds tutorial with worked examples for chemistry students. mass work and key atoms mass and the mole, moles molecules and grams worksheet answer key 1 how many molecules are there in 24 grams of fef ...
This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry.
රසායන විද්යා ශබ්දකෝෂය / Chemistry Dictionary නිර්මාණය: විද්යා ව්යාප්ති අංශය - මූලික අධ්යන ආයතනය මහනුවර
2. Draw the Lewis dot structures for each of the following molecules: a. H 2 S c. SO 3 b. CH 2 Br 2 d. HCN 3. Draw the Lewis dot structure for each of the following polyatomic ions: a. NH 4 + c. PO 4 -3 b. NO 3 - d. CO 3 2- 4. For the following molecules or ions (where the central atom is underlined): i. Draw the Electron dot structure. ii.
Lewis Structure of NO 3-(Nitrite ion). Lewis structure of NO 3-ion is drawn step by step in this tutorial. Total valence electrons of nitrogen and oxygen atoms and negative charge are considered to draw the NO 3-lewis structure. You will every fact of drawing lewis structures from this tutorial which will help you to draw more lewis structures in the future.
How many electron dots are in the Lewis structure of no3 -? There are 24 valence electrons offered for the Lewis structure for NO3-. What is the electron dot structure of NO3? We understand there are 5 valence electrons in nitrogen as well as 6 valence electrons in oxygen.
Lewis dot structures| NO3- ion | nitrate ion | method to draw dot structure| on line textbook,lewis structure of no3- with formal charges, lewis structure of no3- molecule, Lewis structure, resonance structures of no3-, resonance structures of no3-1, resonance lewis structure of no3-, lewis dot structures of no3-, no3-1 lewis dot structure, no3 electron dot structure, lewis dot structure of ...
The Lewis structure of ammonia, NH3, would be three hydrogen atoms bonded to a nitrogen atom in the middle, with a lone pair of electrons. Electron Dot Structure of NH3 by Jeff Bradbury - February 17, - Lewis Electron Dot Structure for ammonia molecule NH3.
tc enter encyclopedia freedom 333 352 dot_clear japanese icon7 cron 296 Forum redhat customer_service 414 index_06 pliki collection 408 1205 Spam ebook moreinfo fd ...
Drawing the Lewis Structure for NO 3-(Nitrate Ion). Nitrates (salts with NO 3-) are frequently used in agriculture as a fertilizer.This is in part to their high solubility in water. There are 24 valence electrons available for the Lewis structure for NO 3-.. Video: Drawing the Lewis Structure for NO 3-
Problem Details. There are 3 Lewis structures for [ NO 3] - draw all three and compare polarity of the 3. Q. Draw the Lewis dot structure for CH2Cl2. Is this molecule polar?
Science Chemistry Q&A Library a) Draw one complete Lewis dot structure for the nitrate ion (NO3 - ) b) Determine the formal charge on each atom in the structure. a) Draw one complete Lewis dot structure for the nitrate ion (NO3 - ) b) Determine the formal charge on each atom in the structure.
Lewis Dot of the Nitrate Ion. NO 3-. Back. 70 More Lewis Dot Structures. The nitrate ion cannot be satisfactorily represented by just one Lewis Dots structure. All the bonds are the same length and must be thought of as a hybrid of multiple resonance structures. YouTube.


![[Download 37+] Resonance Forms Of No3](https://www.flexiprep.com/NCERT-Exercise-Solutions/Chemistry/Class-11/posts/Ch-4-Chem-Bonding-Molecular-Struct-Part-3/Image-showing-resonance-structure-of-NO3.png)



















0 Response to "44 lewis dot diagram for no3"
Post a Comment