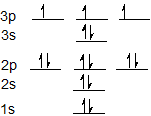
43 orbital diagram of phosphorus
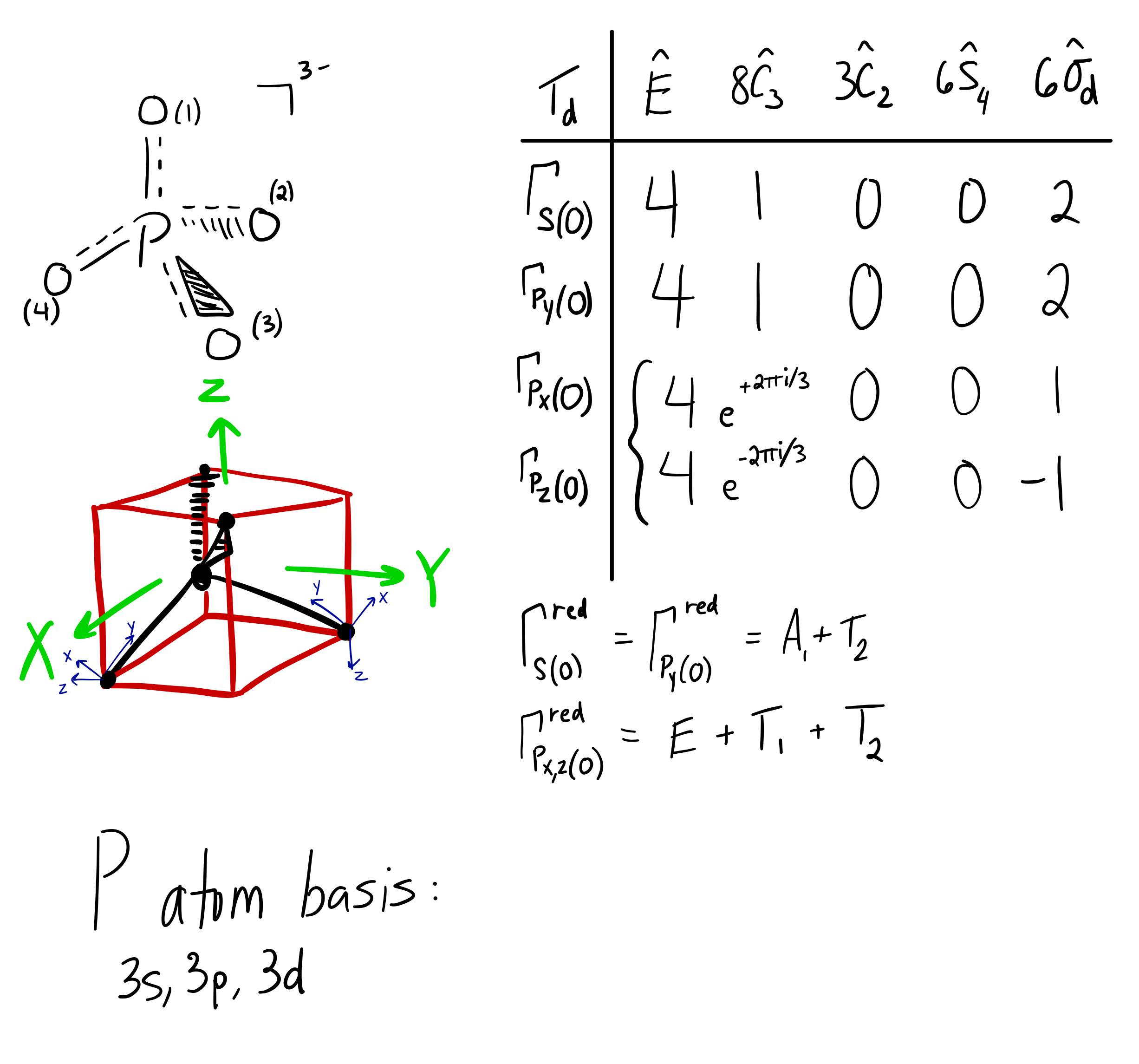
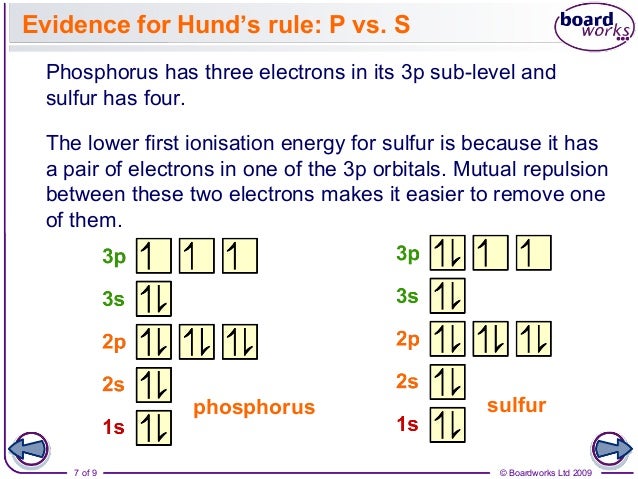
There are three unpaired electrons. As you can see in the electron configuration, the 3p sublevel has room for three more electrons. The orbit filling diagram and the electron dot diagram show the empty spaces for three more electrons and how there are three electrons that aren't paired. HYPERVALENCY OF PHOSPHORUS. Since phosphorus (#"P"#, atomic number #15#) is on the third period of the periodic table, it has access to orbitals of principal quantum number #n = \mathbf(3)#.That means it can use its #3d# orbitals in addition to its typical #3s# and #3p# valence orbitals.. This generates an octahedral molecular and electron geometry.You can see the final shape of this at the ...
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first 10 elements. H #1s^1# He #1s^2# Li #1s^2 2s^1# Be #1s^2 2s^2# B #1s^2 2s^2 2p^1# C #1s^2 2s^2 2p^2# N #1s^2 2s^2 2p^3# O #1s^2 2s^2 2p^4# F #1s^2 2s^2 …
Orbital diagram of phosphorus
Chemistry questions and answers. Question 17.a of 25 Submit Examine the orbital diagram for the ground state electron configuration of phosphorus. Choose the correct orbital diagram for the ground state electron configuration of phosphorus. 1) [Nej 11 3s Зр A) II) [Ne) 11 B) II 3s 3p [Ne] C) III 11 3s 3p D) IV IV) [Ne] 11 + 3s 3p. Orbital diagram for phosphorus (P) Phosphorus (P) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. The valency of the element is determined by electron configuration in the excited state. Clearly, it is a phosphorus sp mixture orbital, with a visible p-type inner lobe, and contains, according to the present calculation, 37% P(3s), 32% P(3p z) and 9% from each fluorine 2p z The highest energy valence-shell MO, labelled as σ * P(3 sp )F(2 p ), has more phosphorus p -character, and contains 19% P(3 s ) and 43% P(3 p z ).
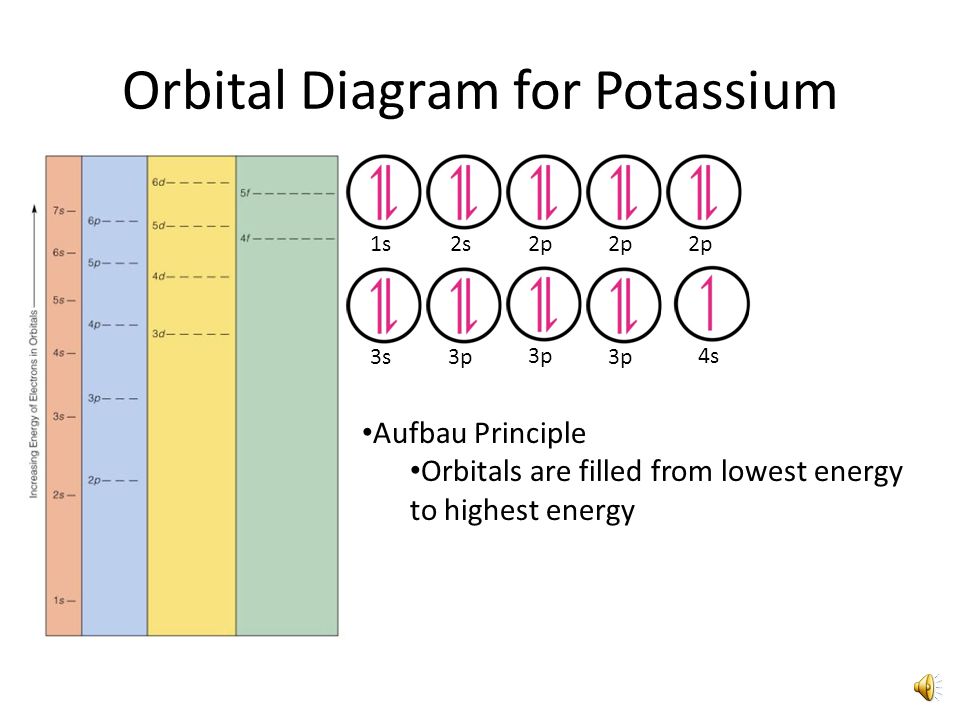
Orbital diagram of phosphorus. The atomic number of phosphorus is 15. It has 5 valence electrons in its valence shell. The orbitals involved in phosphorus atoms are 1s, 2s, 2p, 3s... See full answer below. Become a member and... The remaining one is a non-bonding orbital but doubly field, which denotes the lone pair of phosphorus. Given below is the MO diagram of PF3 taking reference to which you can easily draw for PCl3. A MO diagram helps us to know about the bonding, bond order, bond angle, and bond length of any compound. Nitrogen(N) electron configuration with full orbital diagram. Nitrogen(N) is the 7th element in the periodic table and the first element in group-15. The atomic number of nitrogen is 7 and its symbol is ‘N’. The standard atomic mass of nitrogen is 14.006. The period of nitrogen is 2 and nitrogen is a p-block element. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital.
To write the orbital diagram for the Phosphorus atom (P) first we need to write the electron configuration for just P. To do that we need to find the number... Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. The number of valence electrons impacts on their chemical properties, and the specific ordering and properties of the orbitals are important in physics, so many students have to get to grips with the basics. What is the orbital diagram for phosphorus? Orbital Diagrams: Orbital diagrams show the distribution of electrons in the electron shells and subshells of an atom. A few principles and rules must be... Ab initio LCAO‐MO‐SCF calculations for several typical molecules containing phosphorus have been undertaken to study the role of phosphorus 3d orbitals in the bonding. It is emphasized that the discussion about the 3d orbital participation in bonding should be based on a reasonable choice of basis sets and it seems suitable to choose the atomic orbitals in proper molecular environment as ...
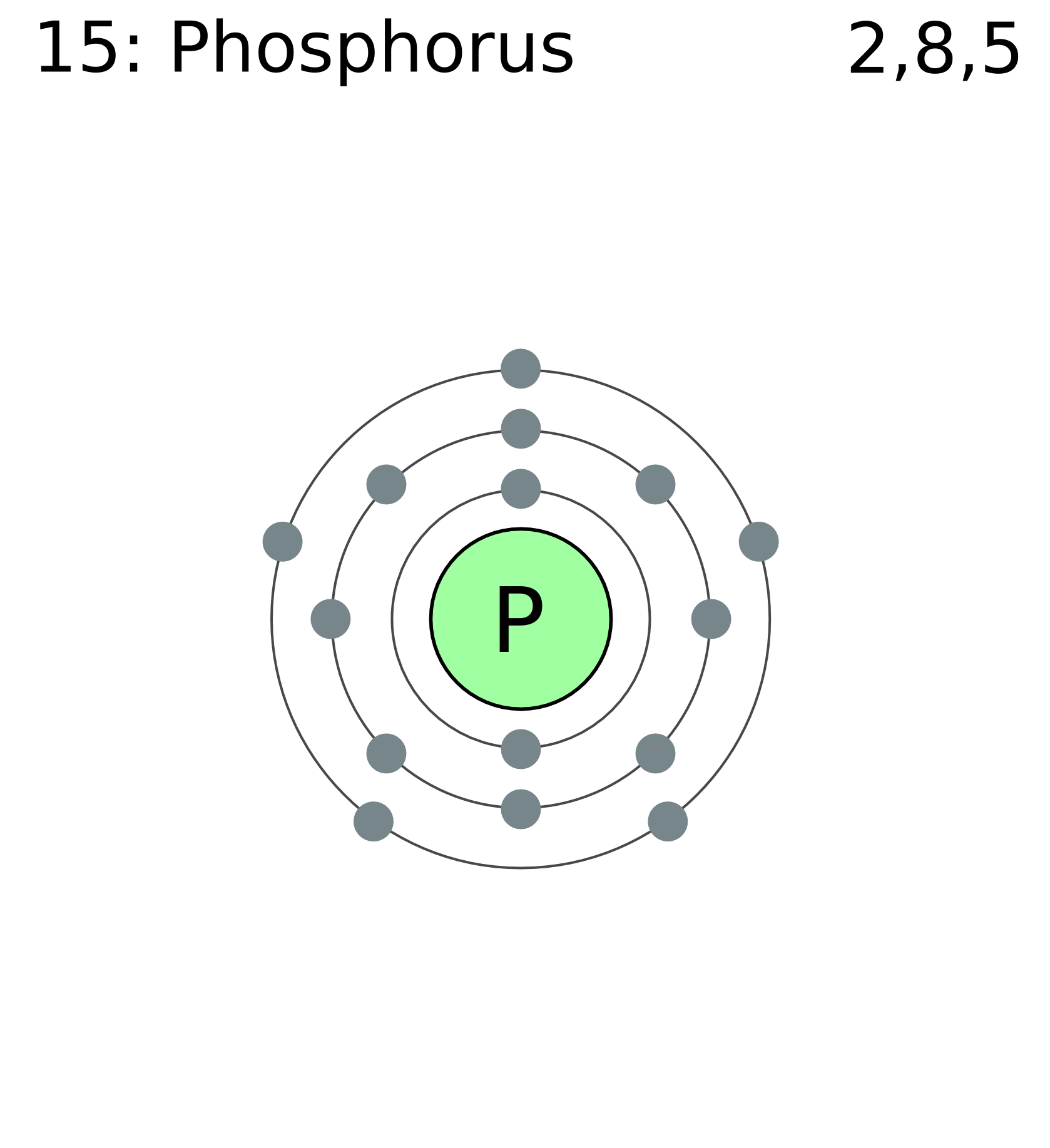
The 15 electrons of the phosphorus atom will fill up to the 3 p orbital, which will contain three electrons: The last electron added is a 3 p electron. Therefore, n = 3 and, for a p -type orbital, l = 1. The ml value could be -1, 0, or +1. The three p orbitals are degenerate, so any of these ml values is correct. A phase diagram combines plots of pressure versus temperature for the liquid-gas, solid-liquid, and solid-gas phase-transition equilibria of a substance. These diagrams indicate the physical states that exist under specific conditions of pressure and temperature, and also provide the pressure dependence of the phase-transition temperatures ... 13. The orbital diagram for a ground-state oxygen atom is. 14. The orbital diagram for a ground state carbon atom is. 15. Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) germanium C) selenium D) tellurium E) none of these. 16. What is the atomic structure of phosphorus? Diagram of the nuclear composition and electron configuration of an atom of phosphorus-31 (atomic number: 15), the most common isotope of this element. The nucleus consists of 15 protons (red) and 16 neutrons (blue). 15 electrons (green) bind to the nucleus, successively occupying available electron shells (rings).
In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons.

Mobile Delousing Unit, Truck Equipment, Plan of Operation and Erection Procedure, Presentation Drawing (1943) // Bertrand Goldberg American, 1913-1997
04/08/2017 · But, phosphorus can form maximum of 5 bonds. That is because it has empty 3d orbitals. Figure 4: The orbital diagram for phosphorous and the possible hybridization. Phosphorous can have five bonds by including the 5 electrons in sp 3 d 1 hybridized orbitals. Then, there are no lone pairs on phosphorous. Difference Between Bond Pair and Lone Pair
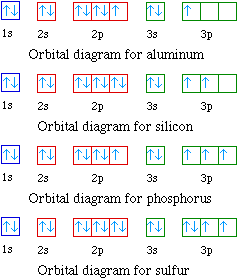
Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine (F) 10: Orbital diagram of Neon (Ne) 11: Orbital diagram of Sodium (Na) 12: Orbital diagram of Magnesium (Mg) 13: Orbital diagram of Aluminum (Al) 14: Orbital diagram of Silicon (Si) 15: Orbital diagram of Phosphorus ...
Structure and bonding. According to X-ray crystallography, [(i-Pr 2 N) 2 P] + is nearly planar consistent with sp 2-hybridized phosphorus center. The planarity of the nitrogen center is consistent with the resonance of the lone pair of the nitrogen atom as a pi bond to the empty phosphorus 3p orbital perpendicular to the N−P−N plane. An idealized sp 2 phosphorus center would expect an N− ...
Moreover, what is the orbital diagram for phosphorus? The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining three electrons. Therefore the Phosphorus electron configuration will be 1s 2 2s 2 2p 6 3s ...

Health Sciences Center, Stony Brook, New York, Sectional Diagram (c. 1974) // Bertrand Goldberg American, 1913-1997
The Magellan spacecraft was a 1,035-kilogram (2,282 lb) robotic space probe launched by NASA of the United States, on May 4, 1989, to map the surface of Venus by using synthetic-aperture radar and to measure the planetary gravitational field.. The Magellan probe was the first interplanetary mission to be launched from the Space Shuttle, the first one to use the Inertial …
A molecular orbital diagram or MO diagram for short is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the Linear combination of atomic orbitals molecular orbital method (LCAO method) in particular.schematron.org: Phosphorus: Orbital and Bonding InfoWhat is the orbital ...
Answer (1 of 3): Here's what I got. Phosphorus has 5 valence electrons, hydrogen contributes one each. Total is 8, so sp3 hybridization. *Without* hybridization would be simple overlap with P orbitals as they would appear in free space. I wasn't about to draw this in Photoshop. ;-) (Feel free...

860-880 North Lake Shore Drive, Electrical Riser Diagram (11/28/1949) // Ludwig Mies van der Rohe (American, born Germany, 1886–1969) Associate Architect: Holsman, Holsman, Klekamp and Taylor (American, 20th century) Associate Architect: Pace Associates (American, 20th century) Structural Engineer: Frank J. Kornacker (American, active 1940s–1950s)
Which ground-state atom has an electron configuration described by the following orbital diagram? A) phosphorus B) nitrogen C) arsenic D) vanadium E) none of these. A) phosphorus. How many unpaired electrons does an atom of sulfur have in its ground state? A) 0 B) 1 C) 2 D) 3 E) 4.

Plate 75 from The Plan of Chicago, 1909: Chicago. Diagram of the City, Showing Complete System of Inner Circuits (1909) // Daniel Hudson Burnham, American, 1846-1912 Edward Herbert Bennett, American, born England, 1874-1954
Electron dot diagram of Carbon atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Carbon, we got to know, it has 4 valence electrons. So, just represent these 4 valence electrons around the Carbon atom as …
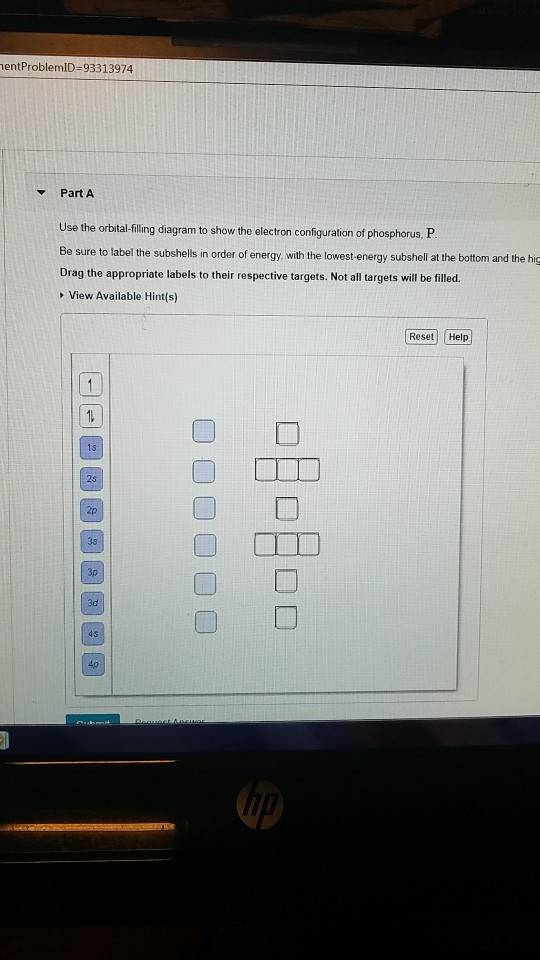
Complete the atomic orbital diagram for the ground-state electronic configuration of phosphorus. 3p C00 Answer Bank Answer Bank Energy 2,0 ; Question: Complete the atomic orbital diagram for the ground-state electronic configuration of phosphorus. 3p C00 Answer Bank Answer Bank Energy 2,0
Irrespective of it, the phosphorus trifluoride (PF3) shows pi bonding characteristics due to sp3 hybridization and back-bonding. The detail of how hybridization is taking place can be studied through the molecular orbital diagram of phosphorus trifluoride (PF3) molecule.
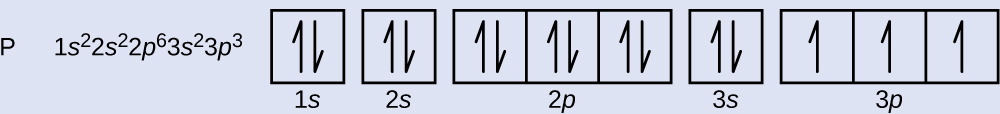
Orbital diagram. Phosphorus electron configuration ← Electronic configurations of elements . P (Phosphorus) is an element with position ... 1s 2 2s 2 2p 6 3s 2 3p 3 Reduced electronic configuration P: [Ne] 3s 2 3p 3. Below is the electronic diagram of the Phosphorus atom Distribution of electrons over energy levels in the P atom 1-st level (K ...
The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms.
phosphorus b. silicon c. carbon d. nitrogen e. oxygen ... The following molecular orbital diagram may be used for the following problems. For oxygen and fluorine, the σ 2p orbital should be lower in energy than the π 2p. However, the diagram will still yield correct bond order and magnetic behavior for these molecules.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
When two atomic orbitals come together to form two molecular orbitals, one molecular orbital will be lower in energy than the two separate atomic orbitals and one molecular orbital will be higher in energy than the separate atomic orbitals. D) Electrons placed in antibonding orbitals stabilize the ion/molecule. E) All of the above are true.
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. Arsenic has 33 electrons, including 3 in its outermost shell. What is the orbital diagram for a ground-state ...
Referring to Figure , draw an orbital diagram to represent those valence orbitals. In writing the electron configuration for Phosphorus the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Phosphorous go in the 2s orbital. The next six electrons will go in the 2p orbital.
Clearly, it is a phosphorus sp mixture orbital, with a visible p-type inner lobe, and contains, according to the present calculation, 37% P(3s), 32% P(3p z) and 9% from each fluorine 2p z The highest energy valence-shell MO, labelled as σ * P(3 sp )F(2 p ), has more phosphorus p -character, and contains 19% P(3 s ) and 43% P(3 p z ).
Orbital diagram for phosphorus (P) Phosphorus (P) excited state electron configuration Atoms can jump from one orbital to another by excited state. This is called quantum jump. Ground state electron configuration of phosphorus is 1s 2 2s 2 2p 6 3s 2 3p 3. The valency of the element is determined by electron configuration in the excited state.
Chemistry questions and answers. Question 17.a of 25 Submit Examine the orbital diagram for the ground state electron configuration of phosphorus. Choose the correct orbital diagram for the ground state electron configuration of phosphorus. 1) [Nej 11 3s Зр A) II) [Ne) 11 B) II 3s 3p [Ne] C) III 11 3s 3p D) IV IV) [Ne] 11 + 3s 3p.

Ball Clock (1948/69) // Attributed to Irving Harper for George Nelson Associates American, born 1916 Made by Howard Miller Clock Company Zeeland, Michigan, founded 1926

Marina City Theater, Chicago, Illinois, Roof and Partial Concrete Frame Development Drawing (1961-1962) // Bertrand Goldberg American, 1913-1997

Plan of Chicago, Chicago, Illinois, Diagram Showing City Growth (1909) // Daniel Hudson Burnham (American, 1846-1912) Edward Herbert Bennett (American, born England, 1874-1954)












/800px-Orbital_representation_diagram.svg-589bd6285f9b58819cfd8460.png)

0 Response to "43 orbital diagram of phosphorus"
Post a Comment