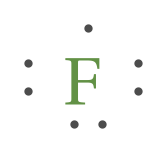
40 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell
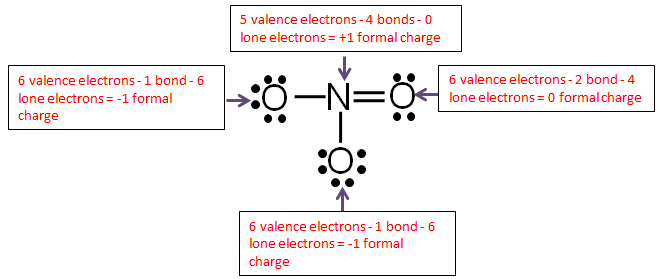
Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: Lewis Structure Examples. The Lewis electron dot structures of a few molecules are illustrated in this subsection. 1. Lewis Structure of CO2. The central atom of this molecule is carbon. Oxygen contains 6 valence electrons which form 2 lone pairs. Since it is bonded to only one carbon atom, it must form a double bond.
Se Lewis Structure. The central atom is selenium, which is bordered on two terminals with hydrogen atoms( in tetrahedral geometry), and two lone pairs on the central selenium atom in the tetrahedral molecular geometry. selenium has six outermost valence electrons, indicating that it possesses six electrons in its outermost shell, whereas hydrogen also has one valence electron in its outermost ...

Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell
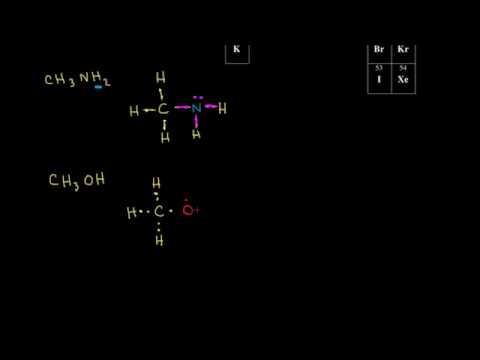
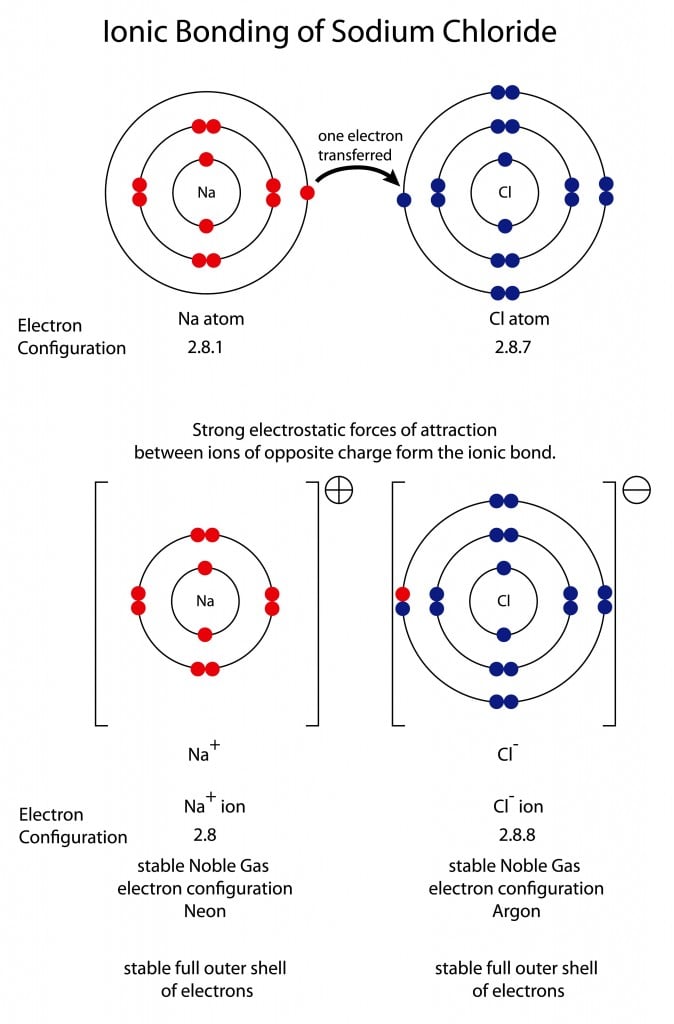
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? When electrons are added to the outermost shell of a carbon atom, it forms; The electrons that occupy the outermost filled shell are called; Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond A Lewis structure is a diagram that shows the bonding between the atoms of a molecule and any possible lone pairs of electrons. To ensure stability, most atoms require an octet - that is, 8 electrons in their outermost electron shell. Hydrogen, however, is an exception. An atom of hydrogen has only one electron shell containing a single electron. Refer to the following Lewis dot diagrams to answer this Question. 5. 6. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? • C • 2: hydrogen carbon 9. 10 A B 21 12 13 :0: :F : 15 16 oxygen flourine hp BADU.
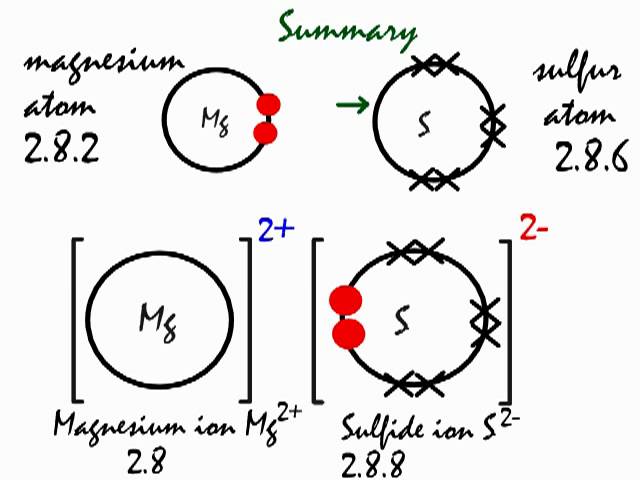
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell. Answer (1 of 2): In exactly the same way as you'd do it for any simple, covalently bonded compound. First you need to know the valency of each element. Then you need to draw a stick model with bonds between the atoms, so that each atom's valency is satisfied (so N has three bonds coming out of i... The Lewis Dot Structure is a visual which represents the outermost shell of electrons, also known as valence electrons, and possible covalent bonds within an atom or molecule. These valence electrons are negatively charged and are attracted to the positively charged nucleus, made up of neutrons and protons. valence electrons (electrons in outermost shell). For example, Sulfur has 6 valence electrons. It needs to gain 2 electrons (2-) to satisfy the octet rule. If sulfur were to bond with calcium (2+)... REVISED KNOWLEDGE: Actual answer to the process quesfions/ focus questions 1. What is Lewis Structure? ' . Lewis structures, also known 'as LeWis dot formulas, Lewis dot structures, electron dot struCtures, or Lewis electron dot VstruCtures, are diagrams that show the bonding between atoms of a molecule, as well as the lone pairs of electrons that may exist in the molecule.
Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? ... So, in order to complete its octet it needs one more electron.1 answer · Top answer: Answer: Option (c) is the correct answer.Explanation:Atomic number of hydrogen is 1 and one dot in its lewis structure displays only one electron. So, ... How does the Lewis dot diagram work? What is a covalent bond? Gilbert Lewis It uses a system of dots to show the valence electrons in a ch… A bond that forms when 2 or more atoms share electrons. 28 Terms lgray5214 Lewis Dot Diagrams Lone pair double bond Bonding pair A pair (2) of electrons not involved in bonding Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell; Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? The electrons that occupy the outermost filled shell are called; What happens to an atom if the electrons in the outer shell are altered? Which electron shell ...
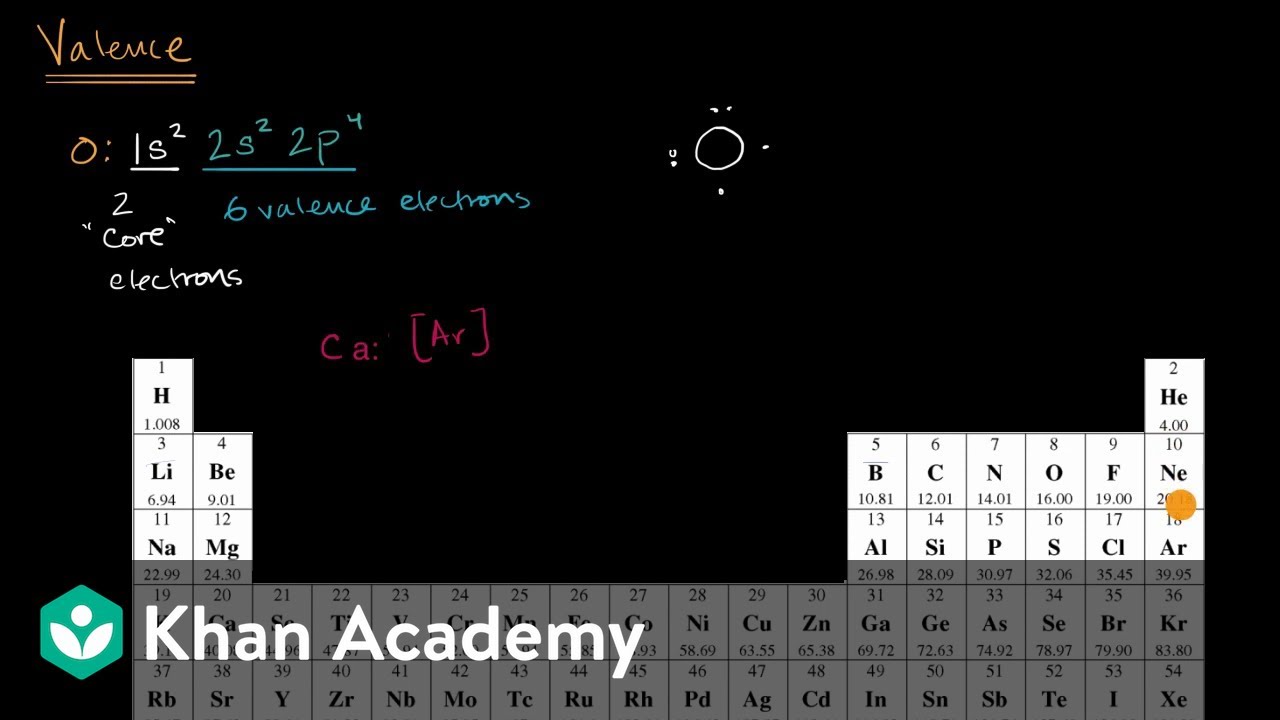
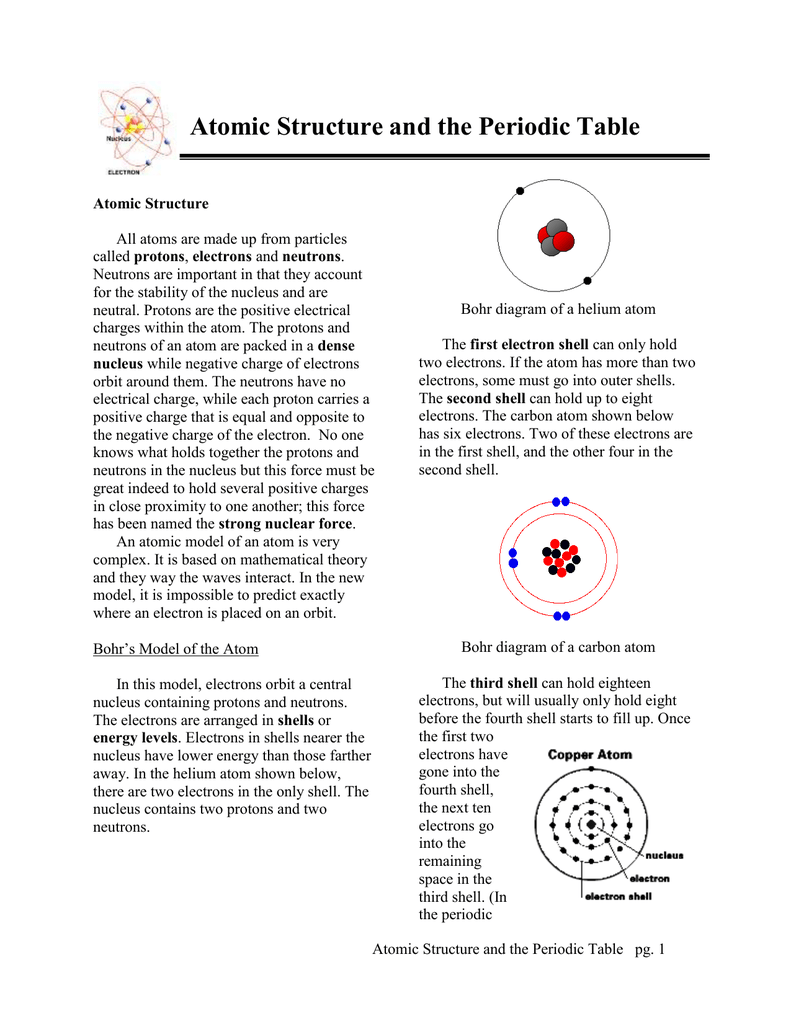
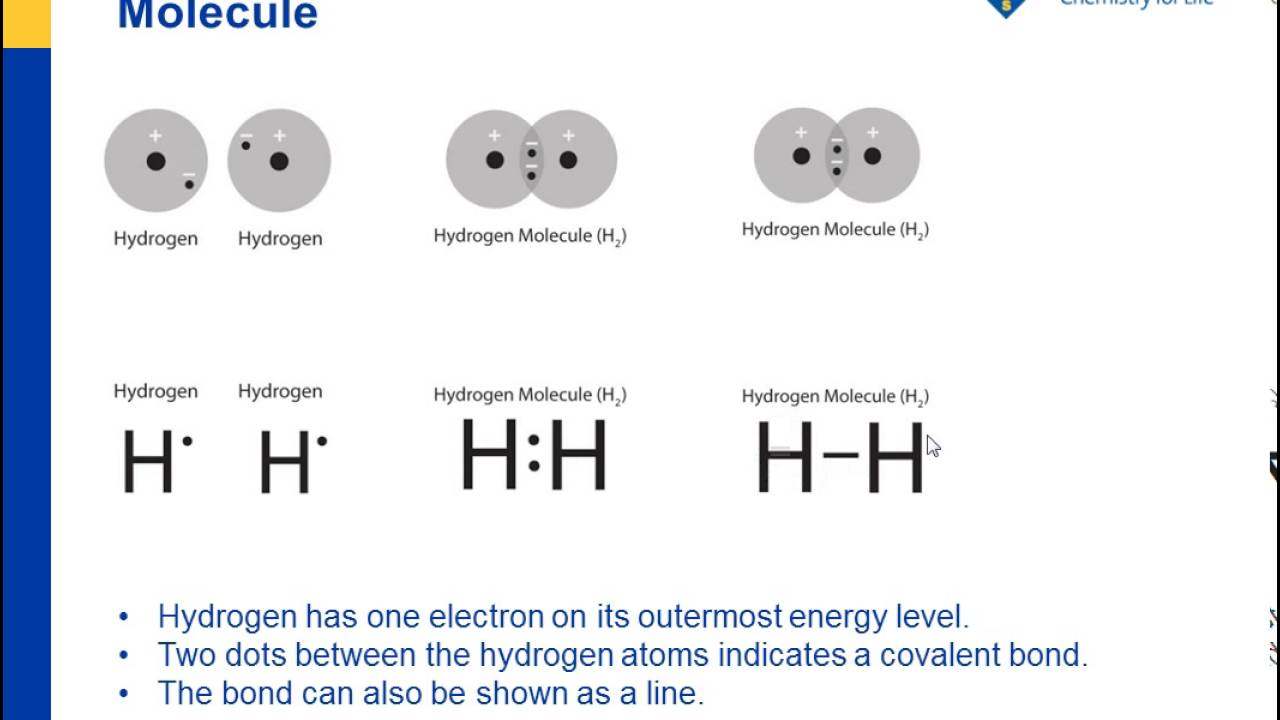
2. Electron Dot Diagrams There is another model called the electron dot or Lewis diagram. This system represents an atom and its valence electrons. The electron dot diagram uses the symbol of the element to replace the nucleus and inner shell electrons. The electrons in the valence shell are shown as dots placed around the symbol. : Mcat Step 3: Find the number of bonding electrons. In this example, once the skeletal structure is specified, a Lewis structure follows fairly readily. Lewis Electron Dot Structure of the molecule: CO (carbon monoxide) The carbon atom has a valency of 4. On the resonance hybrid for NO−2, in between the nitrogen atom and each oxygen atom ... Thus, six electrons (three lone pairs) remain. These lone pairs must be placed on the Xe atom. This is acceptable because Xe atoms have empty valence shell d orbitals and can accommodate more than eight electrons. The Lewis structure of XeF 2 shows two bonding pairs and three lone pairs of electrons around the Xe atom: The carbon atom needs 8 electrons in its valence shell to complete the octet, if you see the above structure, the carbon central atom is attached with four single bonds, which means, it has already 8 electrons in its outer shell, hence, the carbon atom completed its octet comfortably.
Which Lewis dot diagram shows an atom that needs 2 more electrons in it's outermost shell? Oxygen Which type of energy is the energy that it takes to remove an electron from it's shell?
BHow many electrons should be shown in the Lewis dot structure for hydrogen. Because hydrogen only needs two electrons to fill its valence shell it is an exception to the octet rule. Report an issue. 1 Nitrogen atom needs 3 electrons and all 3 Hydrogen atoms need 1 more electron to get stable.
Hence, we can conclude that out of the given options, lewis dot structure of oxygen atom needs 2 more electrons in its outermost shell. Answer 4.9 /5 73 MissPhiladelphia Lewis dot diagram is also termed as the Lewis structure. It illustrates the bonding between atoms of a molecule. (H) hydrogen needs 1 more electron (C)carbon needs 4 more electrons
'Refer to the following Lewis dot diagrams to answer this Question: Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? H ...4 answers · Top answer: skirts the electron configuration when were asked to determine the Louis structure for the Adam ...
Follow some steps for constructing the lewis dot structure of NCl3 1. Count total valence electron in NCl3. In the first step, we have to calculate how many valence electrons are available for NCl3. Because valence electron helps to know how many electrons are present in the outermost shell of an atom.
Oxygen is reactive because it needs to gain two more electrons to fill its outermost shell while neon's outermost shell is completely full of electrons. In more technical terms, neon has an octet...
To write a Lewis symbol for an atom, place the atom's chemical symbol in the center. This symbol will be the atomic core and will represent the nucleus and inner electrons for that atom. The valence electrons will be represented as a dot. Dots are placed around the atomic core singly for the first four electrons.
For example, each atom of a group 14 element has four electrons in its outermost shell and therefore requires four more electrons to reach an octet. These four electrons can be gained by forming four covalent bonds, as illustrated here for carbon in [latex]\ce{CCl4}[/latex] (carbon tetrachloride) and silicon in SiH 4 (silane).
Which Lewis dot diagram shows an atom that needs two more electrons in its outermost shell? 1 See answer Advertisement Advertisement JubileeK is waiting for your help. Add your answer and earn points. KnSomers KnSomers D, fluorine needs two more electrons on the outermost shell. I hope this helps! Advertisement Advertisement
Play this game to review Chemistry. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Preview this quiz on Quizizz. ... Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? Unit 7 Test Review DRAFT. 10th grade. 11 times. Chemistry. 63% average accuracy. 5 months ago ...
Carbon has four valence electrons and it needs four more electrons to complete eight electrons to its valence shell. So, in Lewis dot structure of the carbon atom is the central atom and two H and two F atoms are bonded to it with single covalent bonds. The molecular geometry of the molecule is tetrahedral (in box form).
30 Questions Show answers. Q. How many electrons should Oxygen have around its Lewis dot model? Which of the following shows a correct Lewis dot structure? Which element could X represent? Q. How many electrons should Carbon have around its Lewis dot model? Q. According to the octet rule most elements need _______ valence electrons.
Refer to the following Lewis dot diagrams to answer this Question. 5. 6. Which Lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? • C • 2: hydrogen carbon 9. 10 A B 21 12 13 :0: :F : 15 16 oxygen flourine hp BADU.
A Lewis structure is a diagram that shows the bonding between the atoms of a molecule and any possible lone pairs of electrons. To ensure stability, most atoms require an octet - that is, 8 electrons in their outermost electron shell. Hydrogen, however, is an exception. An atom of hydrogen has only one electron shell containing a single electron.
Which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell? When electrons are added to the outermost shell of a carbon atom, it forms; The electrons that occupy the outermost filled shell are called; Which lewis electron-dot diagram represents a molecule having a nonpolar covalent bond







/ScreenShot2018-11-19at11.40.52PM-5bf3909a46e0fb00510dbd6d.png)











0 Response to "40 which lewis dot diagram shows an atom that needs 2 more electrons in its outermost shell"
Post a Comment