Secondly, activation energy (Ae) is usually higher for endothermic reactions than exothermic reactions so the 'distance' between the peak (energy level of ...1 answer · 3 votes: There are a few key differences between the two energy profile graphs. Firstly, for an endothermic ... 9 Jul 2021 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Let's consider a general reaction where a reactant or set of reactants, A, is transformed into a product or set of products, B. The diagram below is called a ...
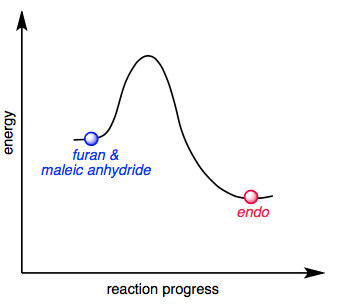
Reaction coordinate diagram endothermic vs exothermic
The relative energy of the reactants and products, the ΔE on the diagram, determines whether the reaction is exothermic or endothermic. A reaction will be ... An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the ... Endothermic Versus Exothermic Reactions ... An endothermic reaction is one where at the end of the reaction, energy is put into the molecule instead of released.
Reaction coordinate diagram endothermic vs exothermic. Endothermic Versus Exothermic Reactions ... An endothermic reaction is one where at the end of the reaction, energy is put into the molecule instead of released. An exothermic process releases heat, causing the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the ... The relative energy of the reactants and products, the ΔE on the diagram, determines whether the reaction is exothermic or endothermic. A reaction will be ...

Energy Reaction Coordinate Diagram Endothermic Vs ...

Exothermic Reaction Coordinate Diagram — UNTPIKAPPS

Selectivity in Free Radical Reactions: Bromine vs ...

Printables. Exothermic And Endothermic Reactions Worksheet ...

Reaction Coordinate Diagram Endothermic Vs Exothermic ...
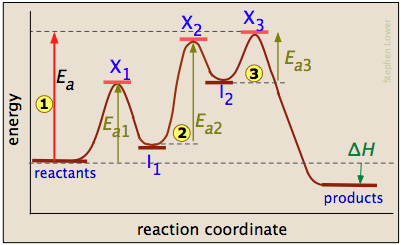
Endothermic Reaction Coordinate Diagram
Endothermic Reaction Coordinate Diagram

Quiz & Worksheet - Energy Reaction Coordinate Diagrams ...

Exothermic or Endothermic reactions - Chemistry Stack Exchange

Energy Diagram For Endothermic Reaction - Atkinsjewelry
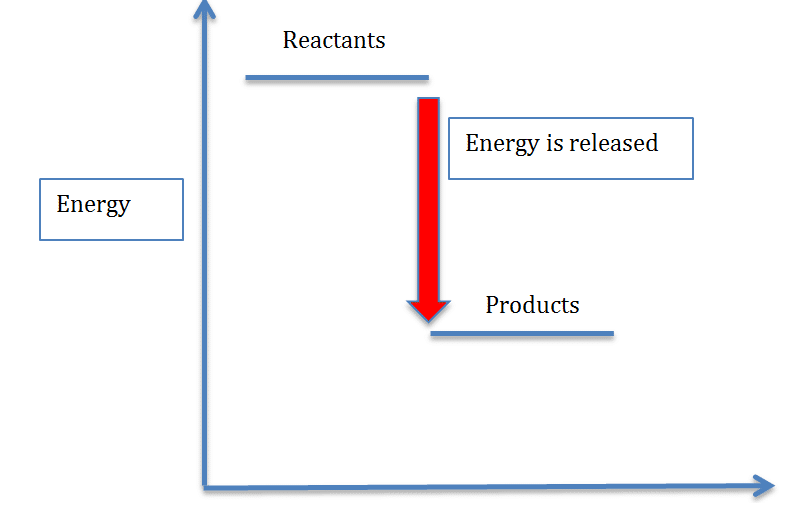
Energy Diagram For Exothermic Reaction - Drivenheisenberg
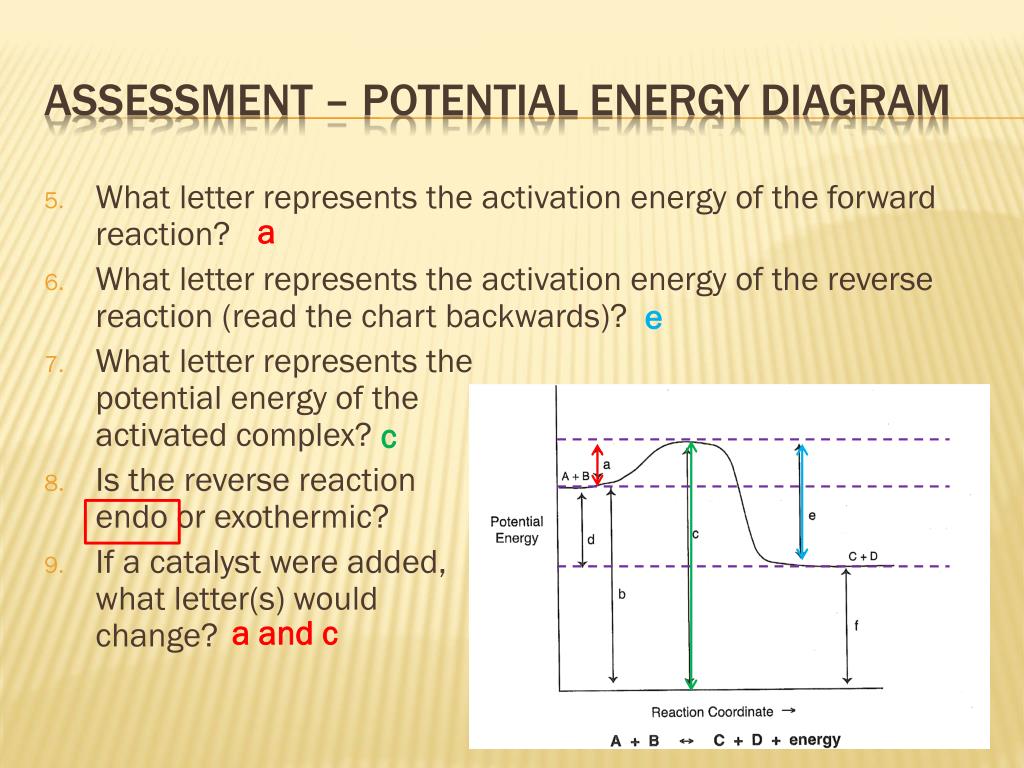
Qualitative Reaction Energy Diagram Exothermic - Diagram Media

35 Select The Potential Energy Diagram That Best ...

Exothermic Reaction Coordinate Diagram — UNTPIKAPPS
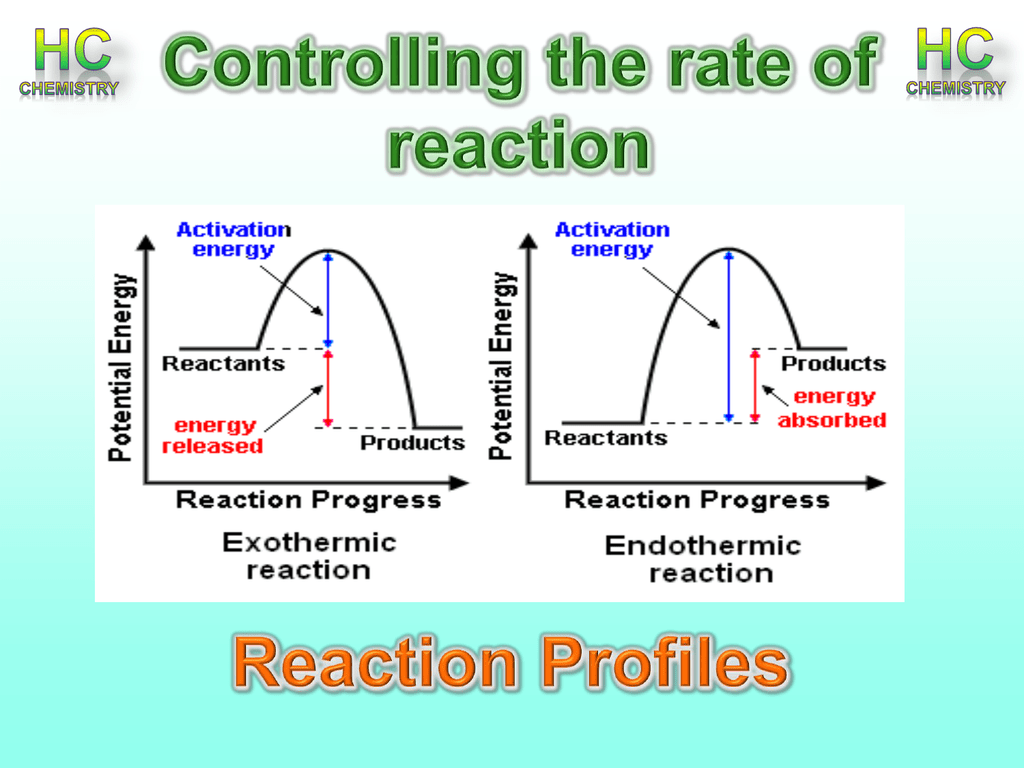
Energy Diagram Endothermic And Exothermic Reaction ...

CHEMISTRY 2 flashcards | Quizlet
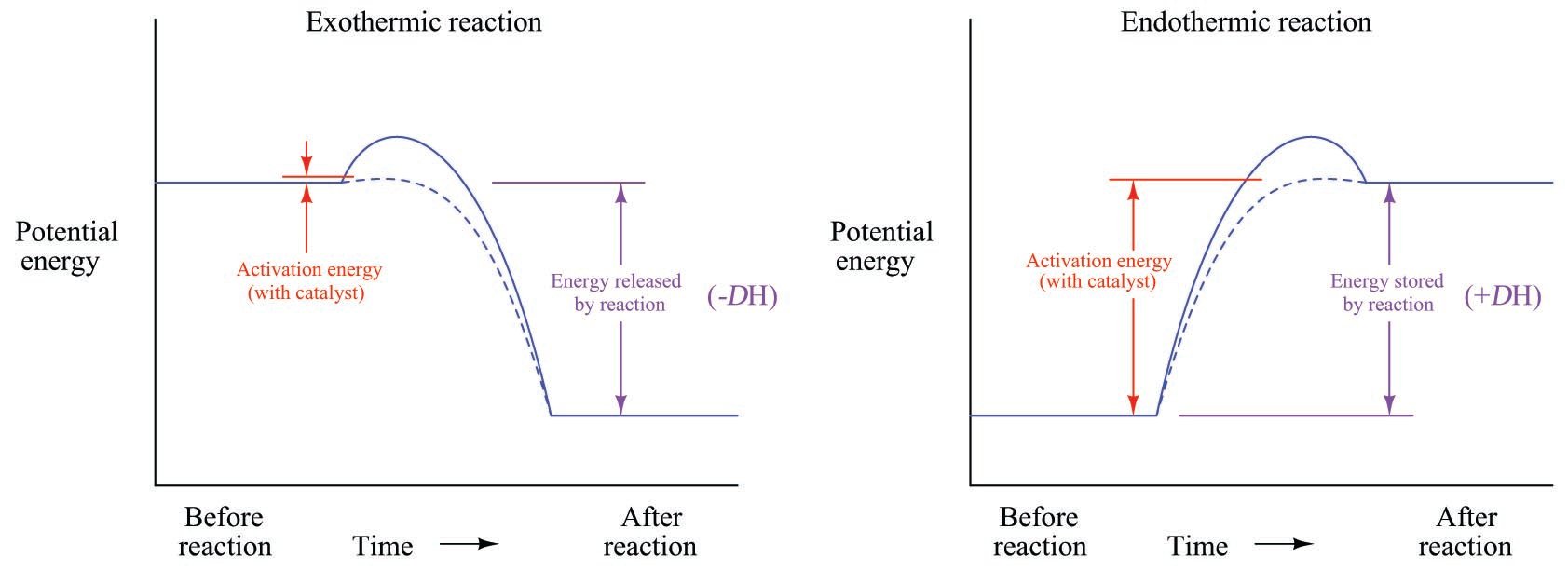
Endothermic Reaction Diagram Labeled - Diagram Media
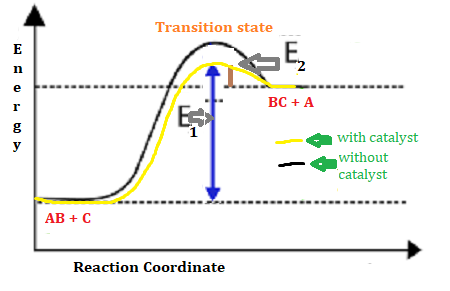
Draw a reaction profile (diagram of energy vs reaction ...

Ch 15 Web

organic chemistry - Why is free radical chlorination ...
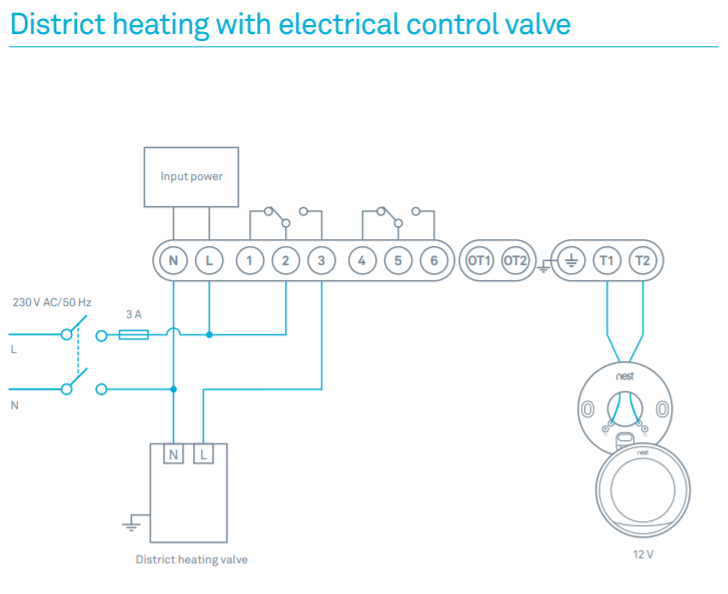
Reaction Coordinate Diagram Endothermic
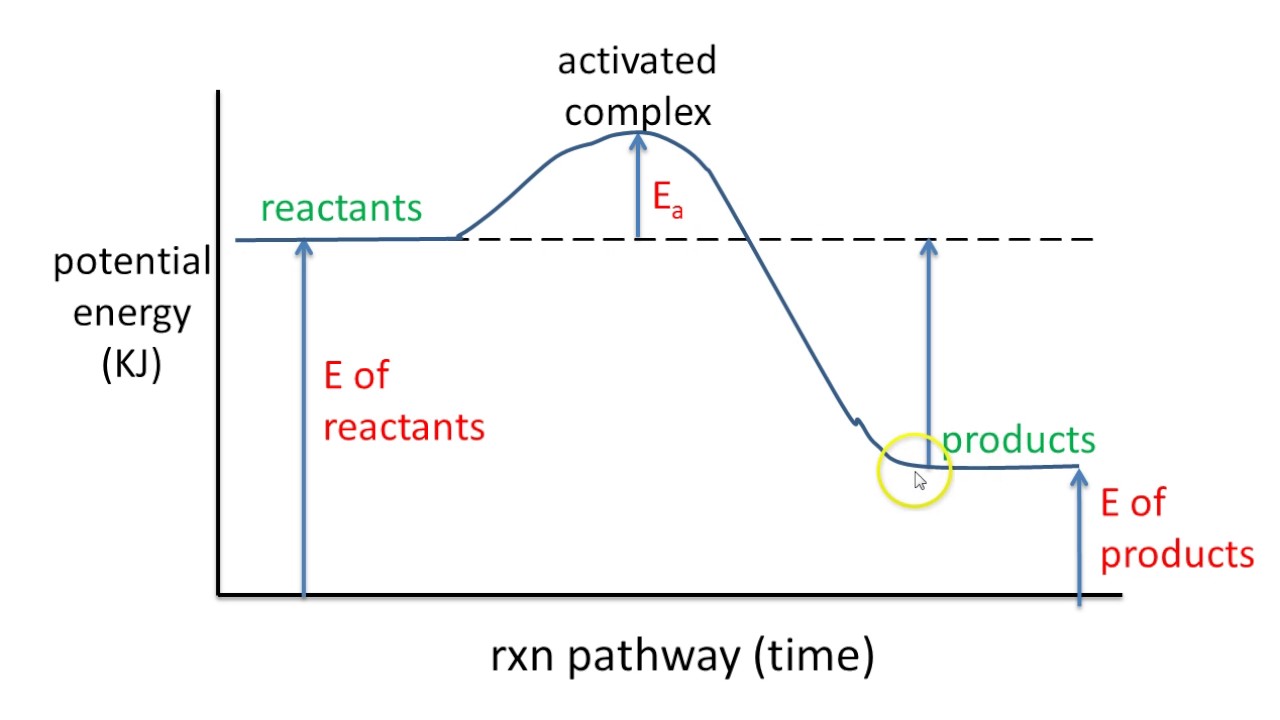
Energy Diagram For Exothermic Reaction - Atkinsjewelry
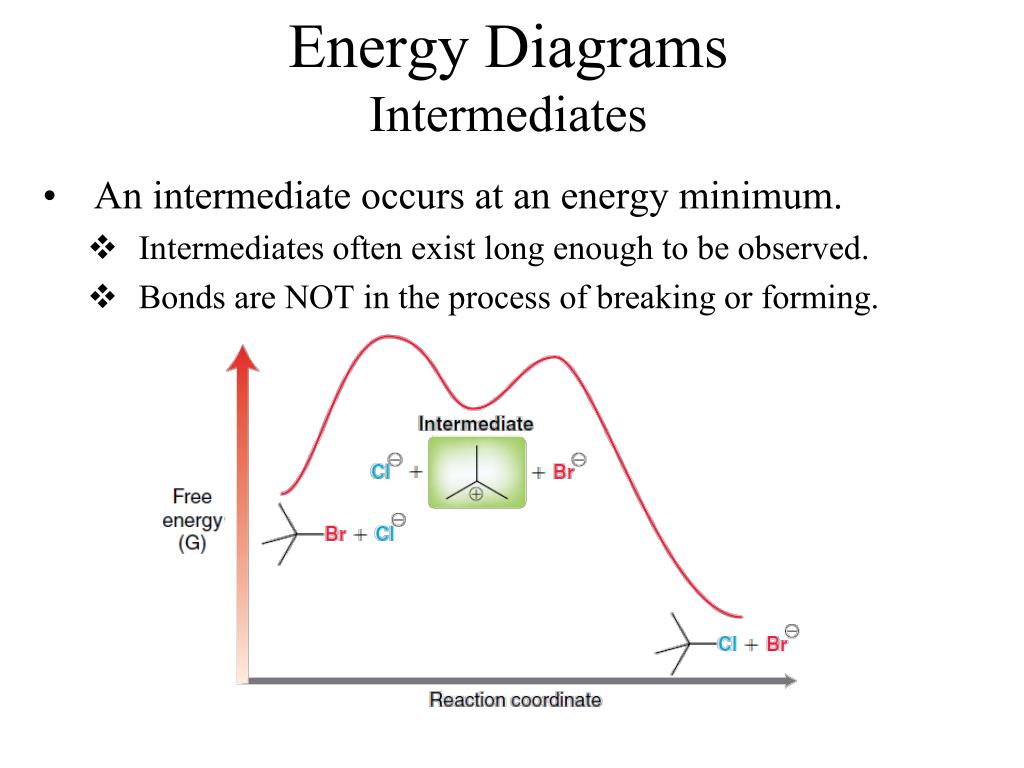
Energy Reaction Coordinate Diagram Endothermic Vs ...

Endothermic Reaction Activation Energy Diagram - Diagram Media

Exothermic Reaction Coordinate Diagram — UNTPIKAPPS

Potential Energy Diagrams - Chemistry - Catalyst ...

Reaction Coordinate Diagram Endothermic — UNTPIKAPPS

Hammond’s Postulate in Organic Chemistry Reactions ...
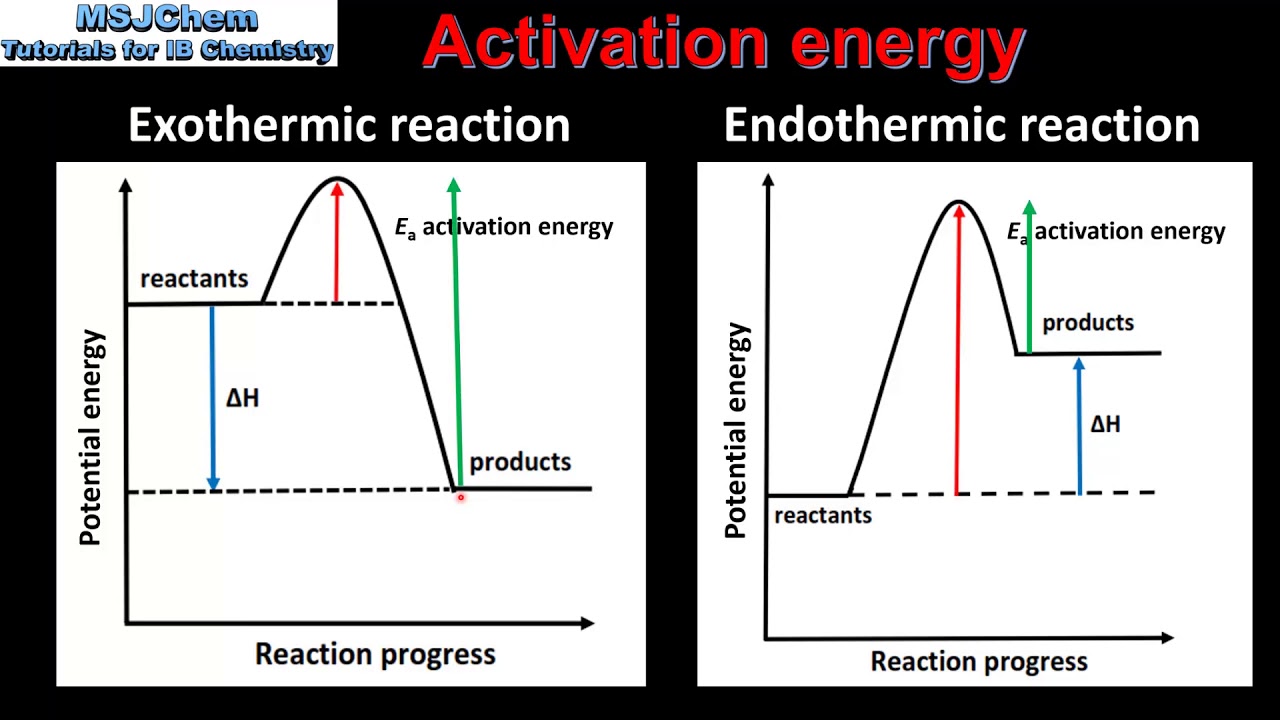
Endothermic Reaction Activation Energy Diagram - Diagram Media

Click On The Point Of The Energy Diagram That Represents ...
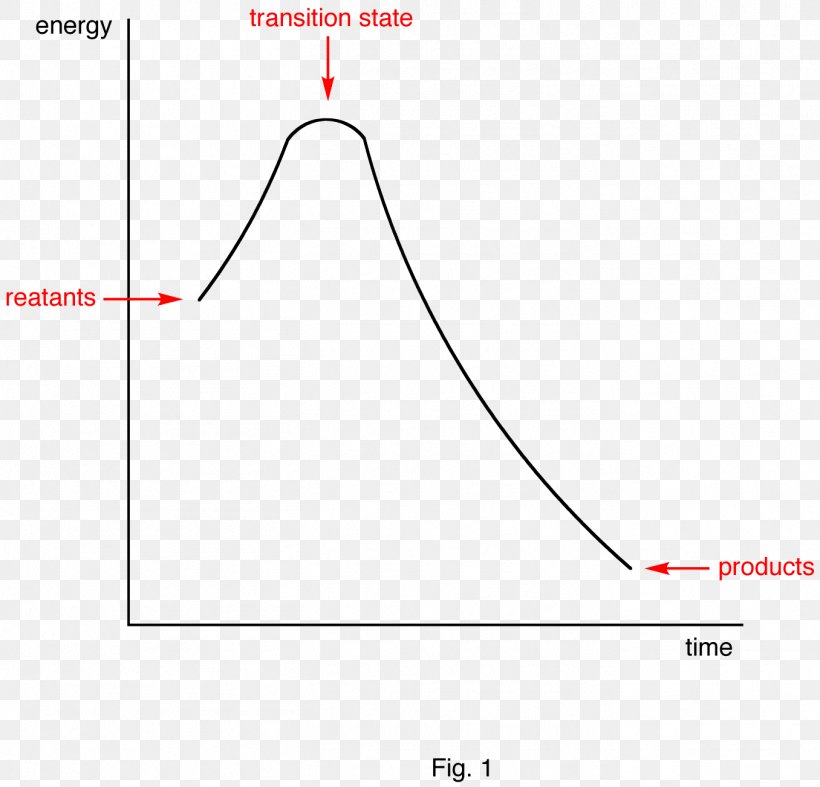
30 Energy Diagram Endothermic And Exothermic Reaction ...

Chemical Forums: Equilibrium Question
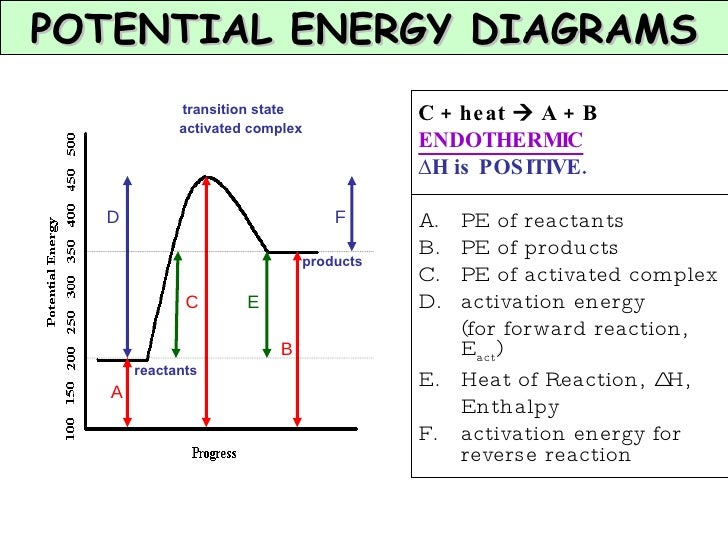
Endothermic Reaction Coordinate Diagram

Enthalpy Profile Diagram For Endothermic Reaction ...

Reaction Coordinate Diagram Endothermic Vs Exothermic ...

Reaction Coordinate Diagram Endothermic Vs Exothermic

Exothermic Reaction Coordinate Diagram - Diagram Media

Endothermic Reaction Coordinate Diagram - Diagram Media

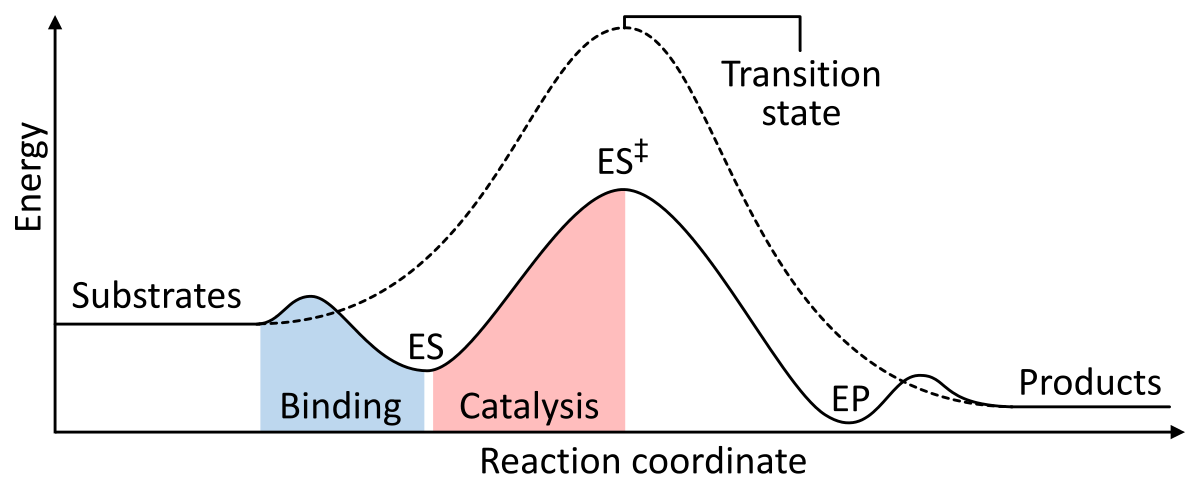
Catalysis

Exothermic Reaction Coordinate Diagram — UNTPIKAPPS




























0 Response to "40 reaction coordinate diagram endothermic vs exothermic"
Post a Comment