44 bohr diagram for sulfur
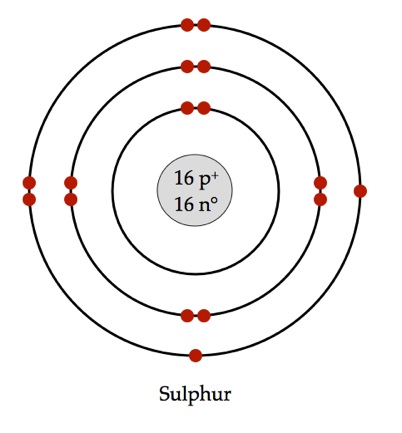
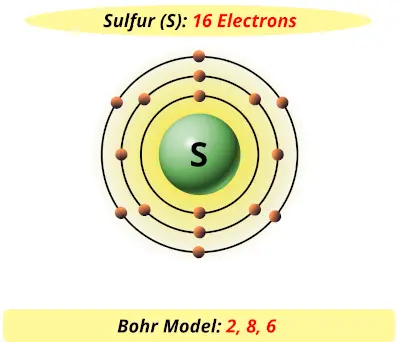
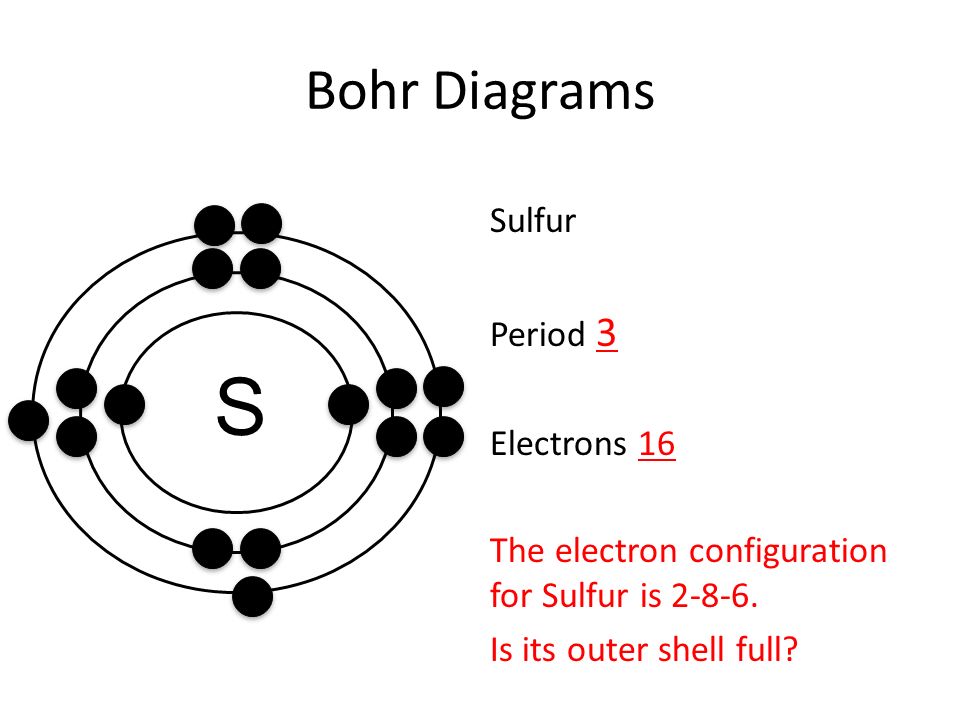
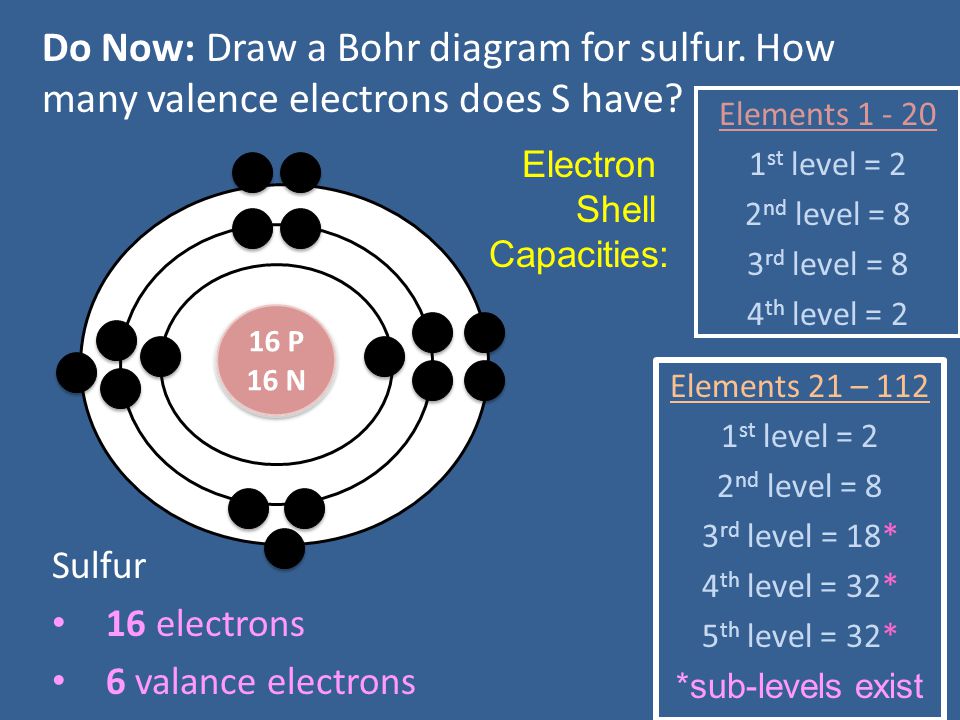
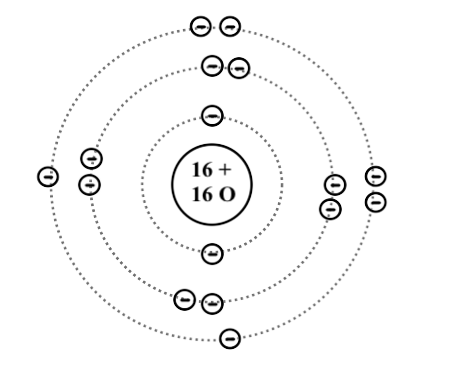
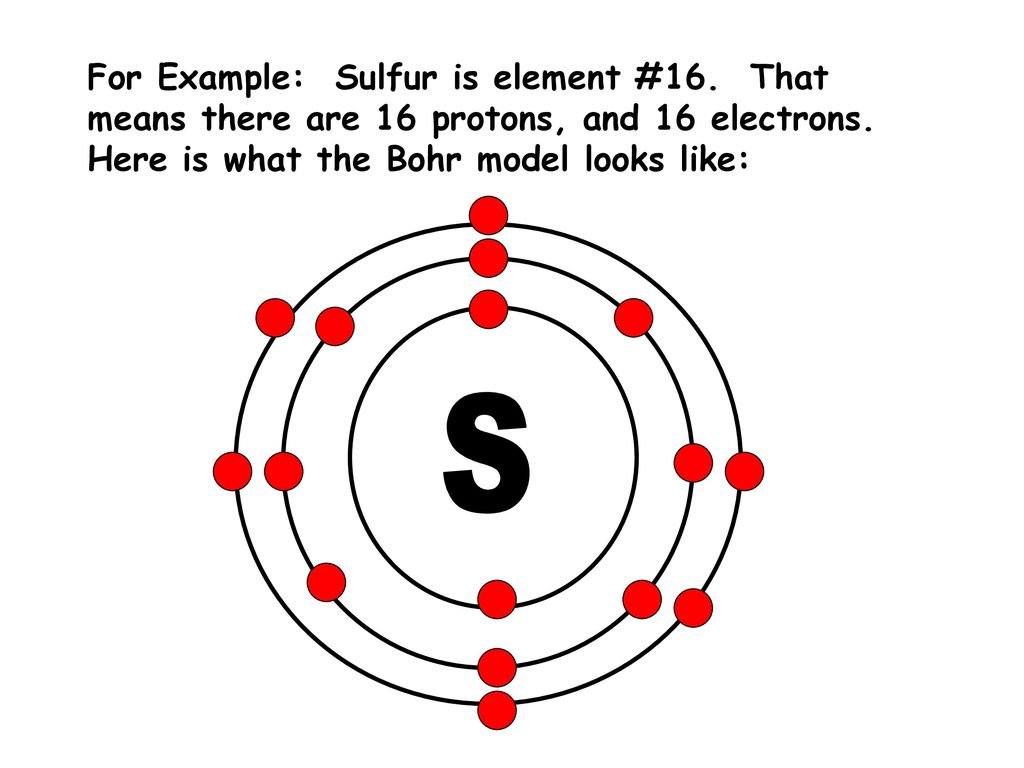
Bohr diagrams are used to introduce. The sulfur atom has 16 protons 16 neutrons and 16 electrons in three different energy levels or orbits. A bohr diagram is a simplified visual representation of an atom that was developed by danish physicist niels bohr in 1913. 1st energy level 2 electrons max 2nd energy level 8 electrons max 3rd levels 18 ...
Sulfur Bohr Model Diagram. Sulfur at Chemical schematron.org Basic Information Atomic Basic Information. Sulfur Atomic Number Electron Configuration. In order to write the Sulfur electron configuration we first need to know the number of electrons for the S atom (there are 16 electrons).
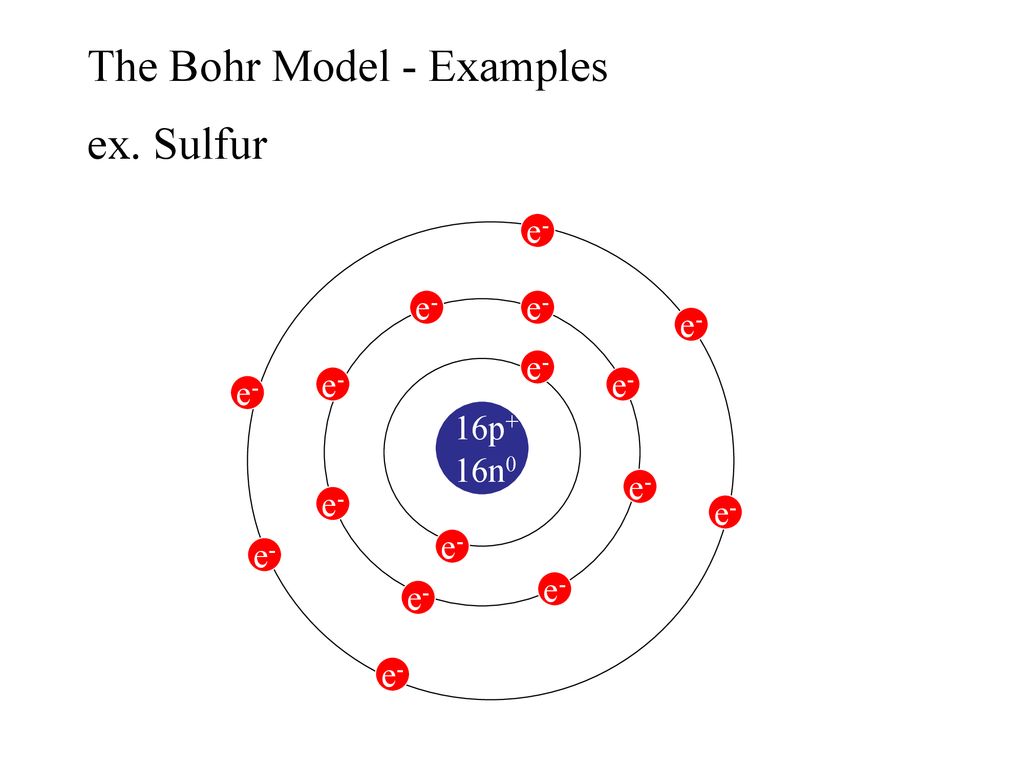
Bohr Diagram Sulfur Shows all electrons Atomic # 16 Atomic Mass — -32 Protons = I (O Neutrons Electron = 15. Calculate the missing information and then draw the Bohr Diagram and Lewis Structure for each element. Atomic # = 3 Mass # = 7 # of N = Atomic # 17 Mass # 35 Atomic # = 10 Mass # = 20 Atomic # = 2 Mass # 4 # of E = d Atomic # 12 Mass ...

Bohr diagram for sulfur
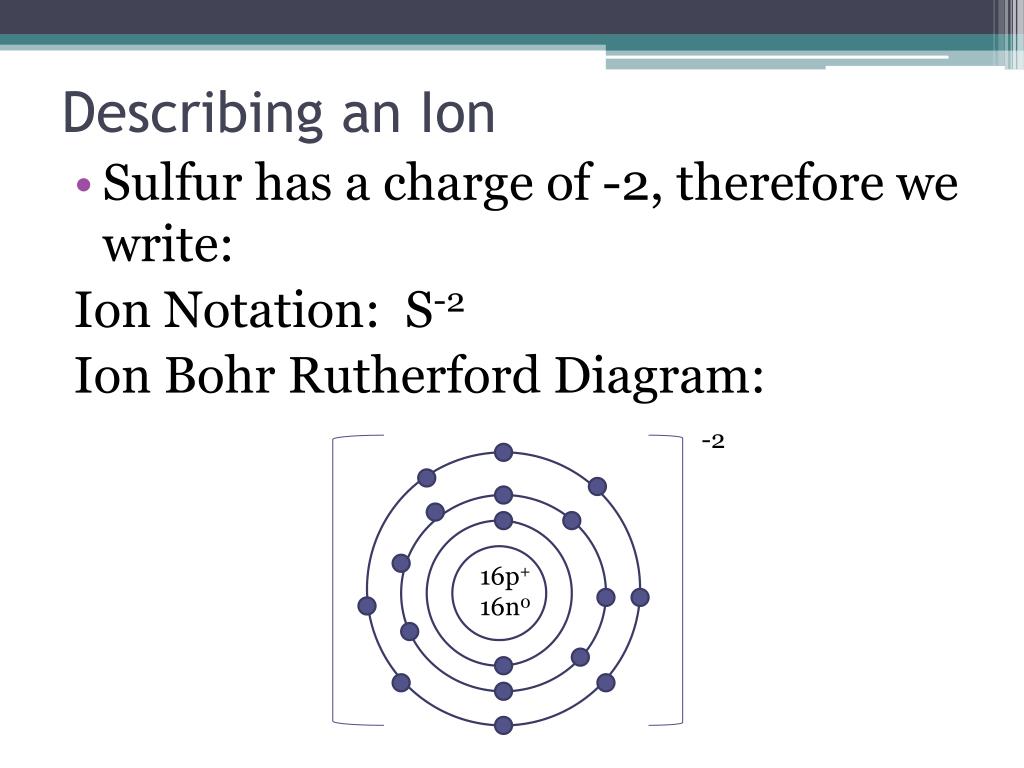
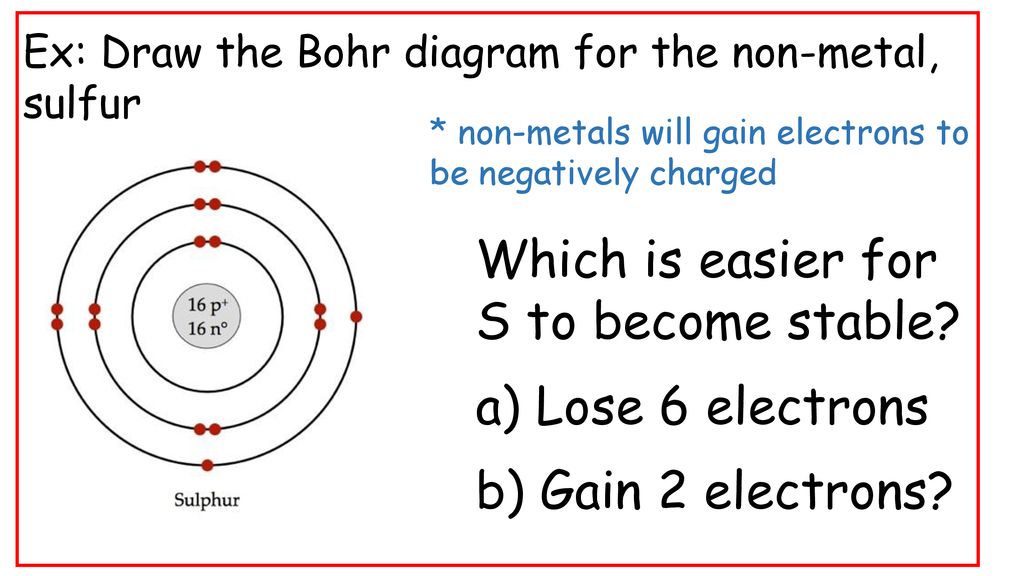
Draw a Bohr-Rutherford diagram of non-bonding sulfur, showing all electrons (core electrons and valence electrons) Draw a molecule of SiS 2, using Bohr-Rutherford diagrams showing all electrons (core electrons and valence electrons). Ensure that all atoms in the molecule have a full valence shell. Show your work.
Created with TouchCast https://itunes.apple.com/us/app/touchcast/id603258418For the interactive version visit:http://touchcast.com/msperrotti/bohr_model_sulfur
Bohr Diagram: The First Element. In order to make a Bohr diagram, you need to know the number of protons, neutrons, and electrons the element has. In this section, we'll show a sample Bohr diagram for hydrogen. H —Hydrogen. 1 proton. 1 electron. 0 neutrons
Bohr diagram for sulfur.
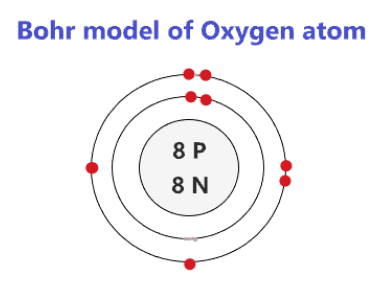
The bohr Rutherford diagram for oxygen has 8 protons and 8 neutrons. There are 2 electrons on the first orbital and six on the second. The bohr Rutherford diagram for oxygen has 8 protons and 8 ...
11+ Sulfur Bohr Diagram. Diagram of connection of the capacitive sensor, for the autolevel function, with an relay module. It is abundant, multivalent and nonmetallic. Sulphur atom - YouTube from i.ytimg.com Draw bohr, electron configuration notation and energy level diagrams for sulfur and vanadium. Each diagram also features the number…
Sulfur / Sulphur has 2 electrons in its first shell, 8 in its second, 6 in its third.Check me out: http://www.chemistnate.com
The Bohr Model of Potassium(K) has a nucleus that contains 20 neutrons and 19 protons. This nucleus is surrounded by four-electron shells named K-shell, L-shell, M-shell, and N-shell. The outermost shell in the Bohr diagram of Potassium contains only 1 electron that also called valence electron.
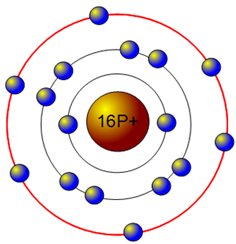
A Bohr diagram is a simplified visual representation of an atom that was developed by Danish physicist Niels Bohr in 1913. The diagram depicts the atom as a positively charged nucleus surrounded by electrons that travel in circular orbits about the nucleus in discrete energy levels.
Bohr Diagram - The Element Sulfur. The Element Sulfur. All About Sulfur! Bohr Diagram.
Bohr Model and Lewis Dot Diagram Worksheet Answers or 50 Best Stock Lewis Dot Diagram for Sulfur Diagram Insp. Bohr model diagrams and lewis dot structures use the information provided for each element to draw bohr model diagrams. Basic atomic structure worksheet answers pdf. What is the charge.
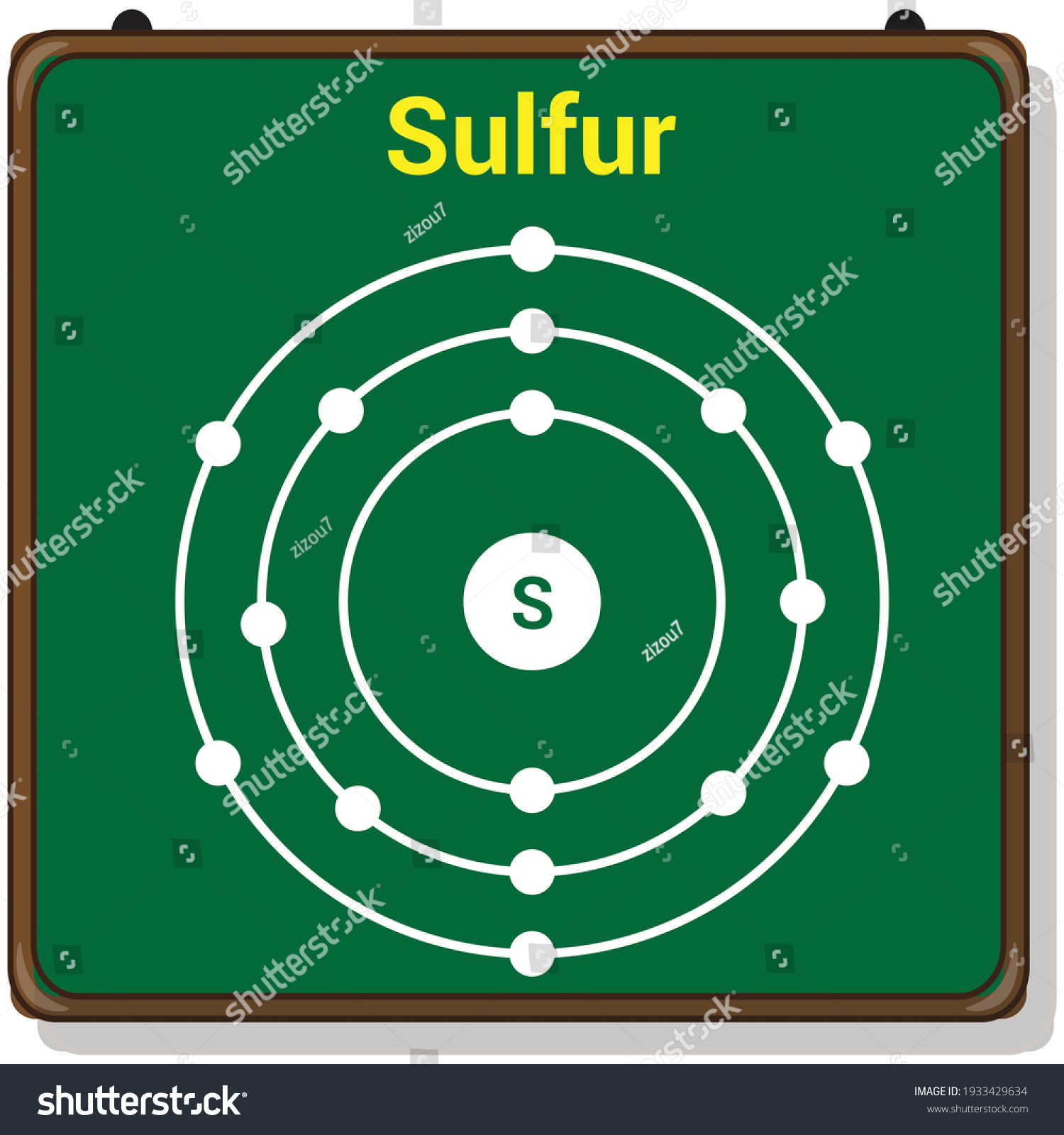
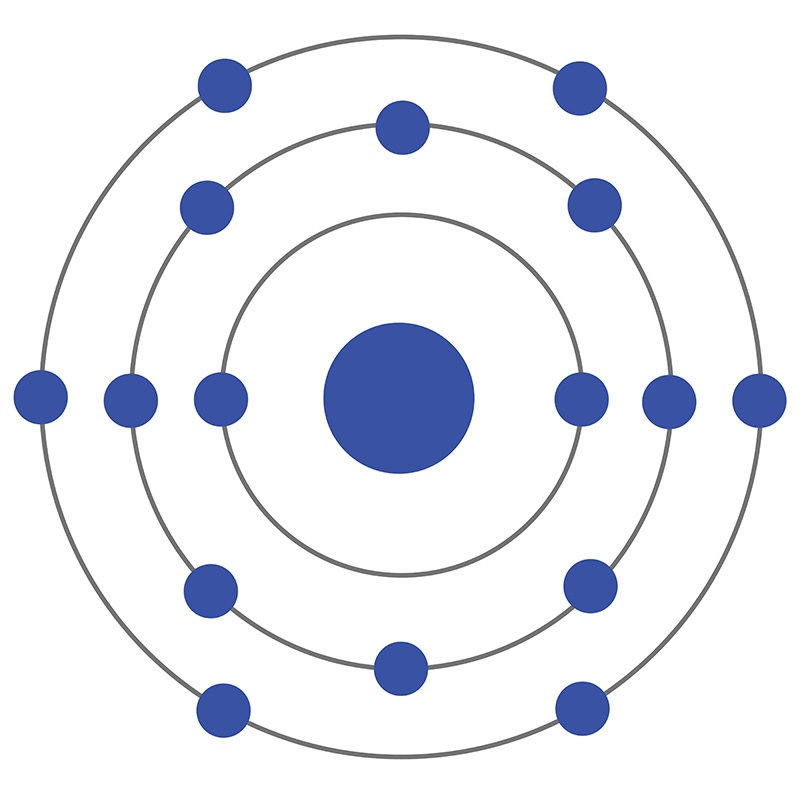
Sulfur Bohr Model Diagram. Sulfur at Chemical schematron.org Basic Information Atomic Basic Information. Name: Sulfur Symbol: S [Bohr Model of Sulfur], Number of Energy Levels: 3. In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,.
Bohr Model of Hydrogen. The simplest example of the Bohr Model is for the hydrogen atom (Z = 1) or for a hydrogen-like ion (Z > 1), in which a negatively charged electron orbits a small positively charged nucleus. Electromagnetic energy will be absorbed or emitted if an electron moves from one orbit to another.
Bohr model of Elements; 1: Bohr model of Hydrogen (H) 1: 2: Bohr model of Helium (He) 2: 3: Bohr model of Lithium (Li) 2, 1: 4: Bohr model of Beryllium (Be) 2, 2: 5: Bohr model of Boron (B) 2, 3: 6: Bohr model of Carbon (C) 2, 4: 7: Bohr model of Nitrogen (N) 2, 5: 8: Bohr model of Oxygen (O) 2, 6: 9: Bohr model of Fluorine (F) 2, 7: 10: Bohr ...
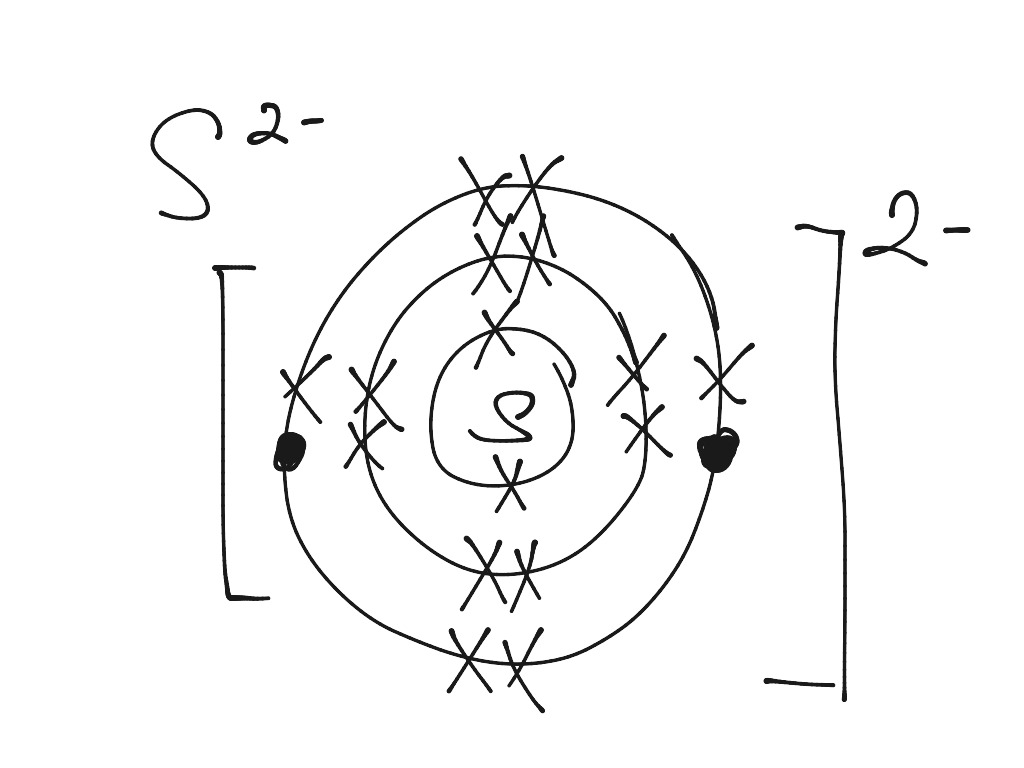
Modify. 2021-11-13. Create. 2004-09-16. Sulfide (2-) is a divalent inorganic anion obtained by removal of both protons from hydrogen sulfide. It is a conjugate base of a hydrosulfide. Chemical groups containing the covalent sulfur bonds -S-. The sulfur atom can be bound to inorganic or organic moieties. Medical Subject Headings (MeSH)
Lewis diagram for Sulfur. Click card to see definition 👆 ... Bohr diagram for Sodium. Lewis diagram for Sulfur. Carbon dioxide. CO2. Sulfur Hexafluoride. SF₆ ...
Bohr diagrams show electrons orbiting the nucleus of an atom somewhat like planets orbit around the sun. In the Bohr model, electrons are pictured as traveling in circles at different shells, depending on which element you have.
What is the Bohr diagram for sulfur? Sulfur has the atomic number 16; therefore, it has 16 protons and 16 electrons. The first energy level will fill up with two electrons. ... Now, since there are only six electrons left and the 3rd energy level has room for 18 electrons, the remaining six electrons will go in the 3rd energy level in the atom ...
The bohr diagram is the diagram of the electrons on the orbital layers of the nucleus of an atom. for potassium, you would put 2 electrons on the first layer, 8 on the second layer, and 9 on the third layer. This is because the atomic number of Potassium (K) is 19, therefore has 19 protons and 19 electrons. How to Make a Model of a Sulfur Atom.
Sulfur Bohr Model Diagram. Sulfur at Chemical schematron.org Basic Information | Atomic Basic Information. Name: Sulfur Symbol: S [Bohr Model of Sulfur], Number of Energy Levels: 3. In the Bohr model, electrons are confined to concentric spheres around the nucleus numbered as n=1, 2, 3,. The sphere n = 1 can accommodate two, the n = Model ...
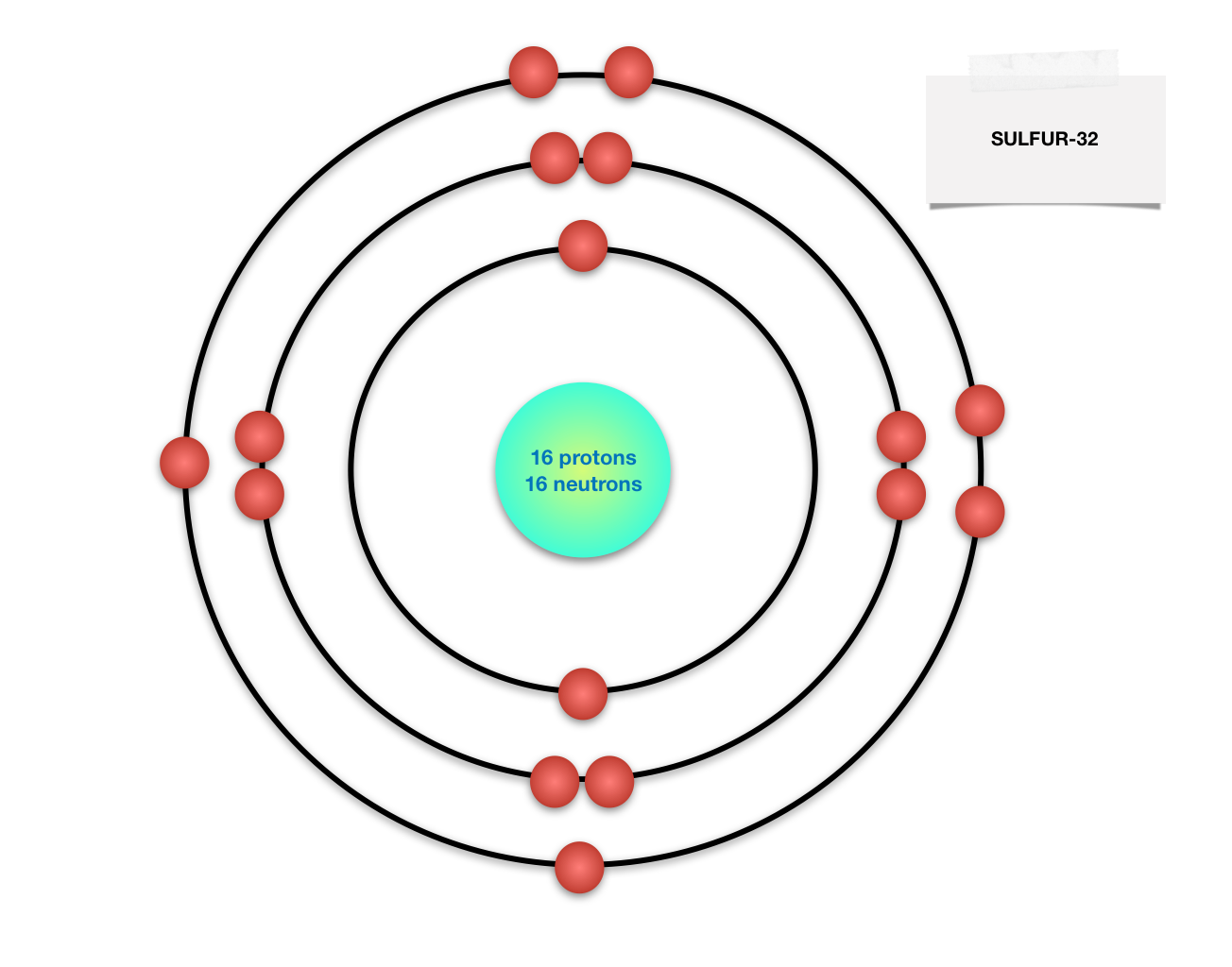
Isotopes of Sulfur (click to see decay chain): 26 S 27 S 28 S 29 S 30 S 31 S 32 S 33 S 34 S 35 S 36 S 37 S 38 S 39 S 40 S 41 S 42 S 43 S 44 S 45 S 46 S 47 S 48 S 49 S : 35 S : Half-life: Fermion, 16p 19n: 87.51157407407 d: Spin 3/2 Parity 1: ... Click any isotope in diagram to see its data.
All About Sulfur! Bohr Diagram. Picture. Powered by Create your own unique website with customizable templates. Get Started. A copper atom is a metal located in group period 4 of the Periodic Table of Elements. Its atomic symbol is Cu. Each atom has 29 protons and electrons. The sulfur atom has 16 protons, 16 neutrons and 16 electrons in three ...
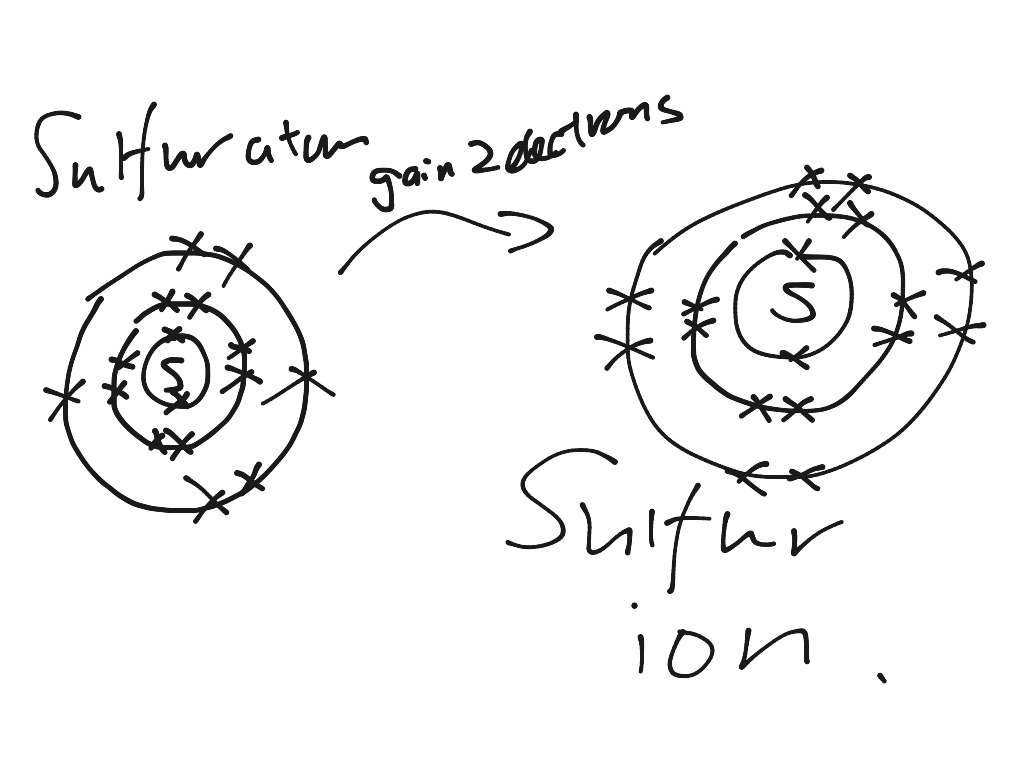
Find step-by-step Biology solutions and your answer to the following textbook question: Draw Bohr-Rutherford diagram for the sulfur-32 atom..
Nov 21, 2020 · Sulfur is a chemical element with atomic number 16 which means there are 16 protons and 16 electrons in the atomic structure. The chemical symbol for Sulfur is S. Sulfur is abundant, multivalent, and nonmetallic. Under normal conditions, sulfur atoms form cyclic octatomic molecules with a chemical formula S8.
5. Below is shown the PES spectrum of sulfur (atomic number = 16). a. Write the full electron configuration of sulfur. b. Label each peak in the spectrum to show which subshell it represents (i.e., 1s, 2s, etc.) c. On the spectrum, sketch in the relative locations and correct peak heights for the spectrum of aluminum (atomic number = 13).
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n. Bohr Diagrams Try the following elements one at a time: a) H b) He
The Bohr Model of Sulfur(S) has a nucleus that contains 16 neutrons and 16 protons. This nucleus is surrounded by three-electron shells named K-shell, L-shell, and M-shell. The outermost shell in the Bohr diagram of Sulfur contains 6 electrons that also called valence electrons.




![How Many Valence Electrons Does Sulfur (S) Have? [Valency of Sulphur]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEhqyh5wZYuX9Q0xdgwqcCUeM_NinkEdU93Rkm7bdQ9Djgn62UjWfslaecZ-uN529KzFC8qhbF3eR5zDmfn7RjNeXFms1omX5cQz_ojZdyyadgH2z4jCV_qokHRM9h-KZZdl1kOn-Jwk_RU/s494/Screenshot+%252872%2529.png)












0 Response to "44 bohr diagram for sulfur"
Post a Comment