43 potential energy diagram for endothermic reaction
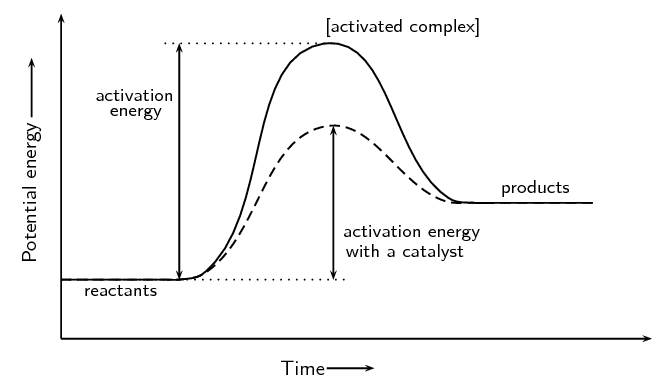
Schematic potential energy diagram showing the effect of a catalyst in an endothermic chemical reaction. Chemistry is the scientific study of the properties and behavior of matter. The loss of this electric potential energy in the external circuit results in a gain in light energy thermal energy and other forms of non-electrical energy. Reactions are classified as either exothermic (H < 0) or endothermic (H > 0) on the basis of whether they give off or absorb heat. Reactions can also be classified as exergonic (G < 0) or endergonic (G > 0) on the basis of whether the free energy of the system decreases or increases during the reaction.. When a reaction is favored by both enthalpy (H o < 0) and entropy (S o > …
A certain reaction happens in two steps. In the first step, A forms B and is slightly endothermic with a high activation energy barrier. In the second step, B forms C + D, which is highly exothermic with a very low activation energy barrier. Sketch the potential energy diagram for this two-step reaction.

Potential energy diagram for endothermic reaction
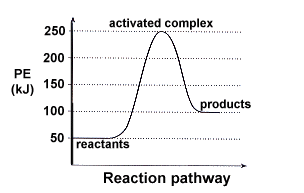
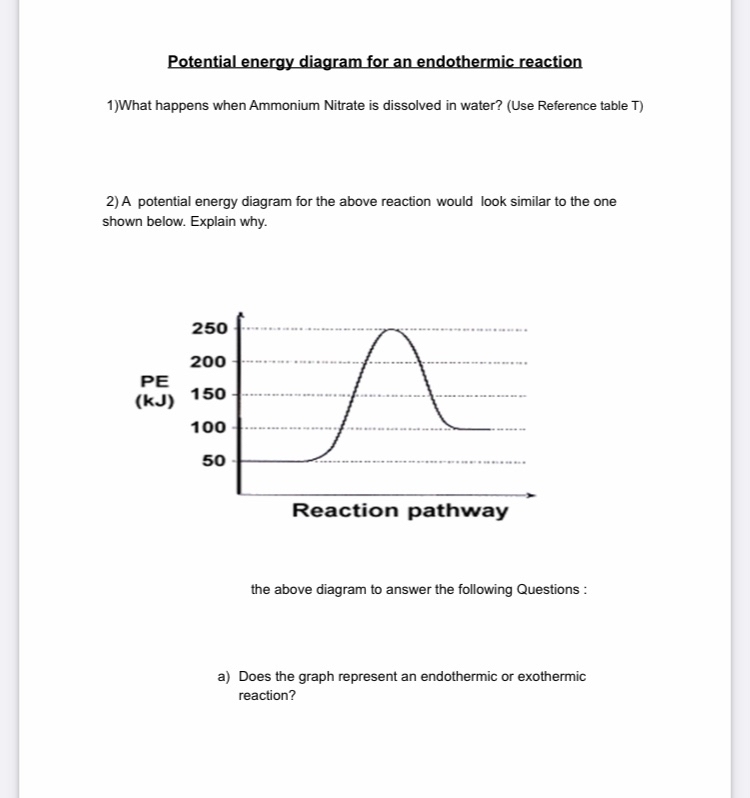
How To Read Potential Energy Diagrams . Endothermic Reaction Lesson Plans Worksheets Lesson Planet . ... Potential Energy Diagrams . Potential Energy Diagrams Ck 12 Foundation . Chemistry 12 . Potential Energy Diagrams Worksheet With Answers Download Printable Pdf Templateroller . Post Navigation. Previous Article Adding Decimals Worksheets Pdf. Old English for "before, in the sight of, in the presence of; as far as; during, before; on account of, for the sake of; in place of, instead of," from Proto-Germanic *fur "before; in" (source also of Old Saxon furi "before," Old Frisian for, Middle Dutch vore, Dutch voor "for, before;" German für "for;" Danish for "for," før "before;" Gothic faur "for," faura "before"), from PIE root *per- (1) "forward," hence "in front of, before," etc. From late Old English as "in favor of." For and fore differentiated gradually in Middle English. For alone as a conjunction, "because, since, for the reason that; in order that" is from late Old English, probably a shortening of common Old English phrases such as for þon þy "therefore," literally "for the (reason) that." For the above Engothermic Potential Energy Diagram: 1. Forward Activation Energy is 250kJ-50kJ = 200kJ. 2. Potential Energy of the Products = 100kJ. 3. Potential Energy of Reactants = 50kJ. 4. Forward Heat of Reaction = 100kJ - 50kJ = 50kJ (Notice the result is positive) Know the different parts of both the Exothermic and Endothermic Potential ...
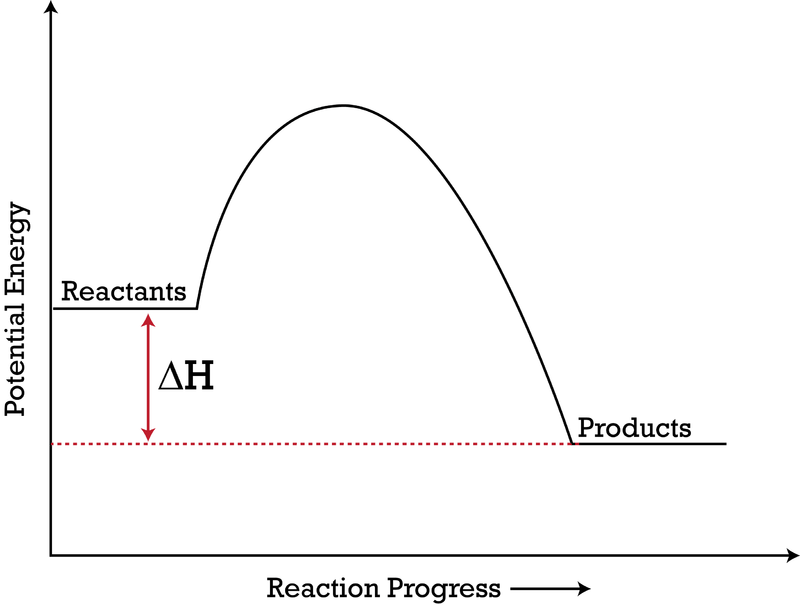
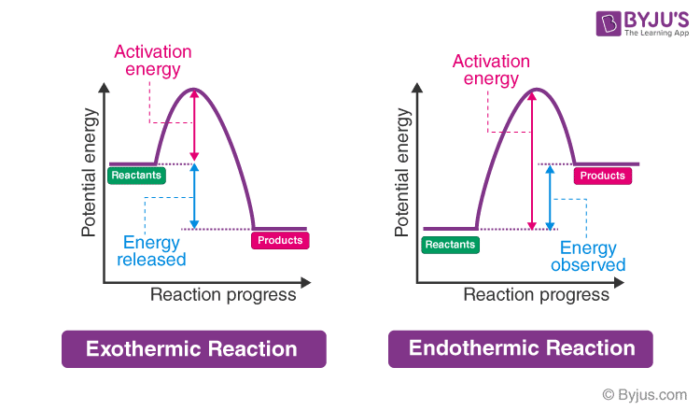
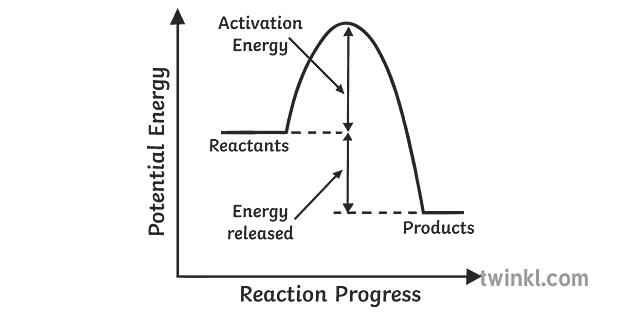
Potential energy diagram for endothermic reaction. Figure 18.4. 1: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and Δ H is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the ... Potential energy diagrams doc 27 kb drawing a potential energy diagram doc 31 kb spontaneous reaction worksheet doc 31 kb chemical reactions video the driving forces doc 26 kb entropy and enthalpy warm up doc 43 kb spontaneous entropy enthalpy and p e diagram questions docx 62 kb. Every chemical reaction will either absorb or release energy. In an exothermic reaction, the products have less potential energy than the reactants had. This is why the potential energy diagram for an exothermic reaction starts at a higher energy value and ends at a lower energy value. In an endothermic reaction, the products end up with more stored potential energy than the reactants. Potential energy diagram worksheet answers 1. δh pep per. The overall difference in potential energy between the products and the reactants. Is the reaction in 6 exothermic or endothermic. Every chemical reaction will either absorb or release energy. For a given reaction the.
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra... Image of a graph showing potential energy in relation to the process of a chemical reaction. In the case of an exothermic reaction, the reactants are at a higher energy level as compared to the products, as shown below in the energy diagram. K the compound ab is a gas and the element c is a solid. Ws 4 potential energy diagrams worksheet 1. Every chemical reaction will either absorb or release energy. Potential energy diagram worksheet 1. A potential energy diagram shows the change in energy during a reaction. Potential energy diagram displaying top 8 worksheets found for this concept. "worthless," 1711, from adjectival phrase (see good (adj.)).
Respond to the three questions below on energy diagrams and submit to your instructor. Consider the potential energy diagram shown below. This graph shows the chemical potential energy in a reaction system over time. The y-axis is potential energy in kilojoules. The x-axis is the reaction progress, or time. Figure 6.6. 1: A potential energy diagram shows the total potential energy of a reacting system as the reaction proceeds. (A) In an endothermic reaction, the energy of the products is greater than the energy of the reactants and Δ H is positive. (B) In an exothermic reaction, the energy of the products is lower than the energy of the reactants ... An energy level diagram. In endothermic reactions the reactants have higher bond energy stronger bonds than the products. Energy diagram for exothermic reaction. In other words the products are less stable than the reactants. The reaction diagrams of exothermic reactions and endothermic reactions are as follows. Apr 09, 2018 · 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
MUST KNOW INFORMATION, Test takes place April 28, 7pm pst should be about 30 min long so im Willing to pay 30 bucks &#x200B; Know the signs that a chemical reaction has occurred. Know how to write a balanced chemical reaction. Know how to write a total ionic chemical reaction and a net ionic chemical reaction. Know the strong acids and the strong bases (these break up in aqueous solutions).Know how to use the solubility Rules to determine if salts are soluble or insoluble. Know the di...
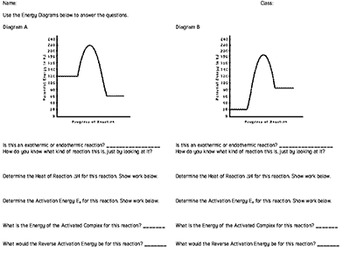
In this chemical reactions worksheet students use two graphs of chemical reactions to identify the activation energy heat content of the reactant and products and determine if the reaction is exothermic or endothermic. For Teachers 6th - 8th. Using the following potential energy graph answer the following questions.
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
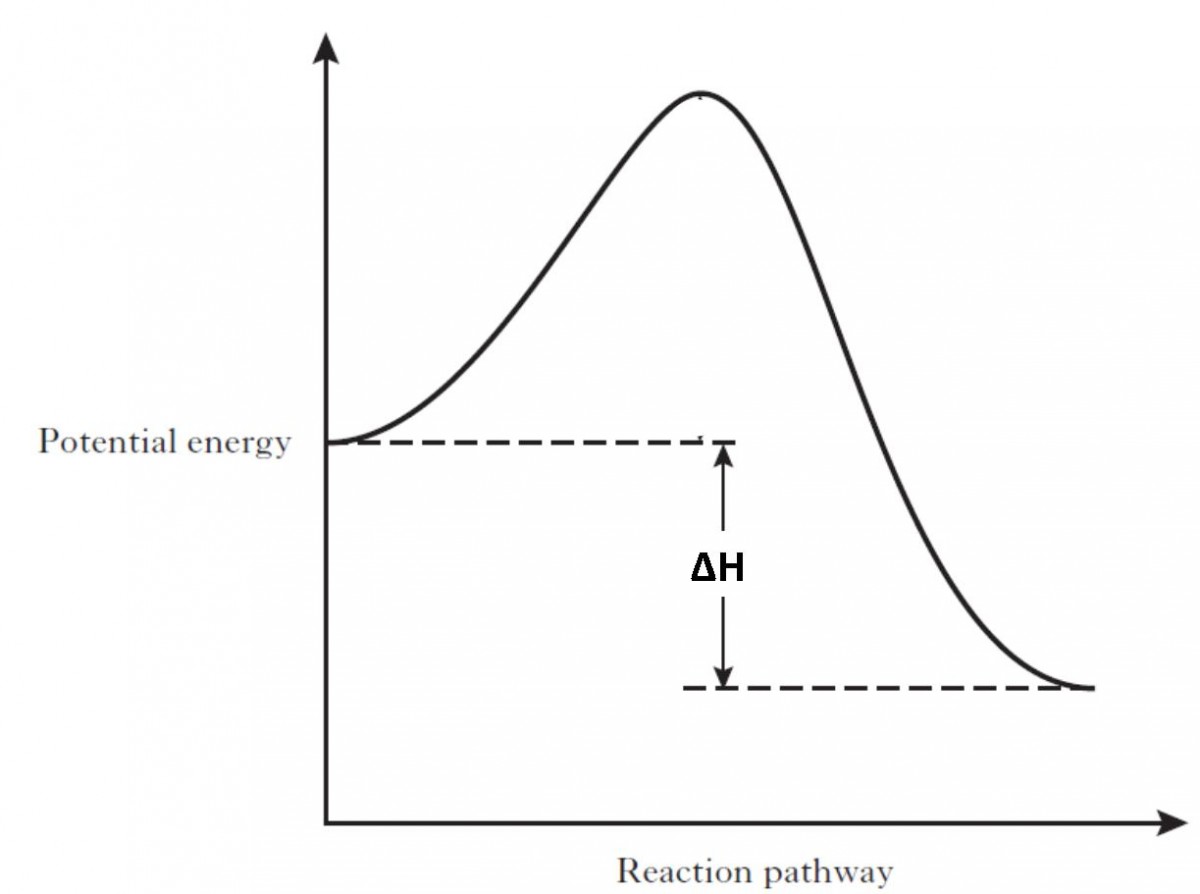
A relationship between q and ΔH can be defined knowing whether q is endothermic or exothermic. An endothermic reaction is the one that absorbs heat and reveals that heat is consumed in the reaction from the surroundings, hence q>0 (positive). If q is positive, then ΔH is also positive, at constant pressure and temperature for the above equation.
Potential Energy Diagram Worksheet Potential Energy Energy Activities Teaching Chemistry. This Is A Short Quiz Worksheet To Check For Student S Understanding Of Kinetic And Potentia Potential Energy Kinetic And Potential Energy Work Energy And Power. Reactions In Which Energy Is Released Are Exothermic Reactions And Those That Take In Heat ...
Potential Energy Diagram Worksheet Potential Energy Energy Activities Teaching Chemistry. This Is A Short Quiz Worksheet To Check For Student S Understanding Of Kinetic And Potentia Potential Energy Kinetic And Potential Energy Work Energy And Power. Reactions In Which Energy Is Released Are Exothermic Reactions And Those That Take In Heat ...
A potential energy diagram plots the change in potential energy that occurs during a chemical reaction. This first video takes you through all the basic parts of the PE diagram. Sometimes a teacher finds it necessary to ask questions about PE diagrams that involve actual Potential Energy values.
1918 (Venn's diagram is from 1904), named for English logician John Venn (1834-1923) of Cambridge, who explained them in the book "Symbolic Logic" (1881).
Jul 06, 2021 · The energy changes that occur during a chemical reaction can be shown in a diagram called a potential energy . [7th]Mathematical Methods for Physicists Arfken.pdf . The activation energy for a reaction is illustrated in the potential energy diagram by the height of the hill between the reactants and the products.
1610s, "an illustrative figure giving only the outlines or general scheme of the object;" 1640s in geometry, "a drawing for the purpose of demonstrating the properties of a figure;" from French diagramme, from Latin diagramma "a scale, a musical scale," from Greek diagramma "geometric figure, that which is marked out by lines," from diagraphein "mark out by lines, delineate," from dia "across, through" (see dia-) + graphein "write, mark, draw" (see -graphy). Related: Diagrammatic; diagrammatically. The verb, "to draw or put in the form of a diagram," is by 1822, from the noun. Related: Diagrammed; diagramming.
Answers: 3 on a question: The diagram shows the potential energy changes for a reaction pathway. (8 points) A curved lined graph is shown. The y axis of the graph has the title Potential Energy. The x axis of the graph has the title Reaction Pathway. The graph curve begins at a higher level and ends at a slightly lower level. A vertical line labeled A, starting from the x axis till the ...
Potential energy diagram worksheet answer. 1232015 33700 PM Company. TRUE - A kgm 2 s 2 is a mass unit times a speed squared unit making it a kinetic energy unit and equivalent to a Joule. Does this potential energy diagram represent an exothermic or an endothermic reaction.
When you draw a Potential Energy Diagrams, you can see the activation energy and if the reaction is exo/endothermic. But some books when explaining the activation energy uses Gibbs only for the y-axis (not potensial), and then you can see if its spontanous or not. The activation energy is some sort of Gibbs isnt it? usually written as Ea or G**? If you are discussing reaction rates is there any difference which diagram you use?
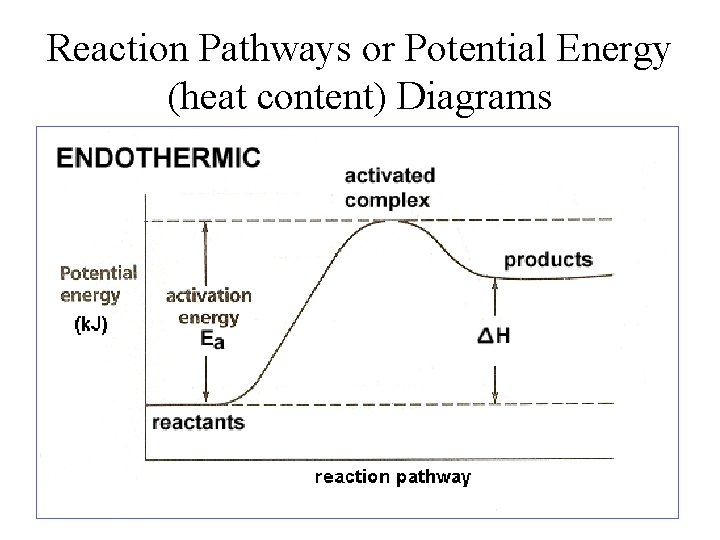
Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. 3.
1869, originally in chemistry, "causing, relating to, or requiring the absorption of heat," from French endothermique (1868, Berthelot); see endo- + thermal. By 1947 in biology, "dependent on or capable of the internal generation of heat; warm-blooded."
Warning: This may be a long post, so bear with me. I currently have some doubts with Kinetics while going over my notes. Consider the following diagram: http://imgur.com/sgCWwFn 1. Why do we have that from the transition state (as it is a "combined" form of the reactants, shouldn't there be "bonds" as well?) we have a potential energy decrease by going to the products? Wasn't it that bond breaking was an endothermic process? If so, why is the case that the potential energy of the system is goi...
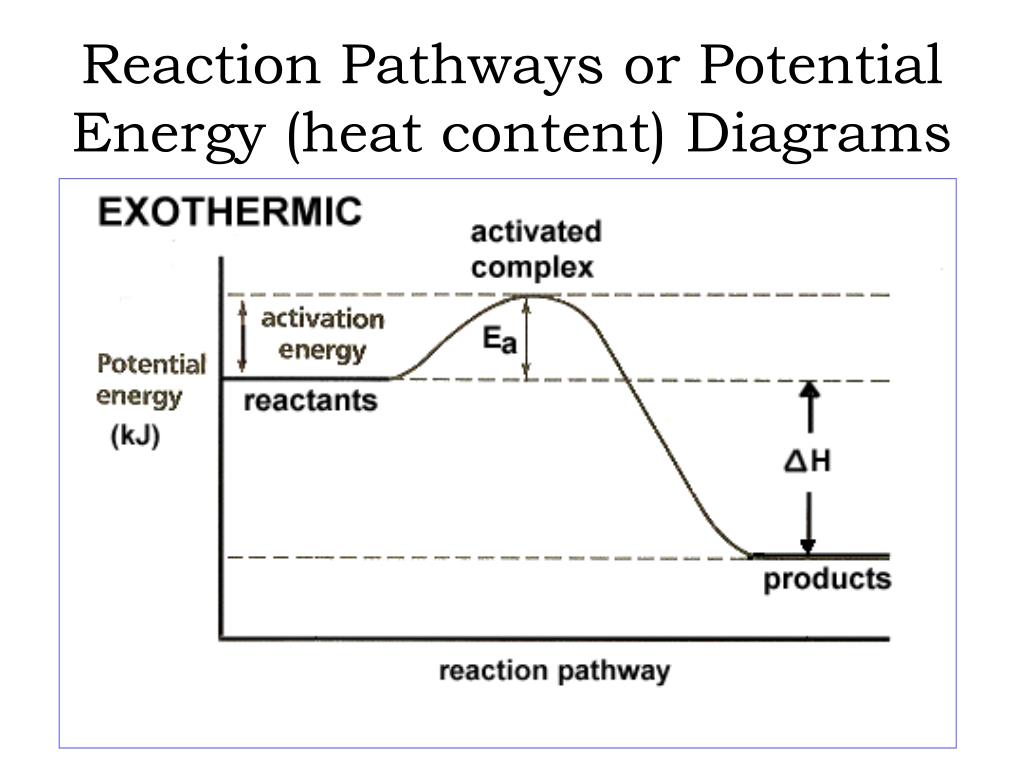
An energy profile is a diagram representing the energy changes that take place during a chemical reaction. Enthalpy change, ΔH, is the amount of energy absorbed or released by a chemical reaction. On an energy profile, the enthalpy change for the reaction is measured from the energy of the reactants to the energy of the products. Exothermic ...
Since the potential energy of the products is higher than the potential energy of the reactants, the reaction is endothermic and the enthalpy (E(C)-E(A)) has a positive sign.The activation energy (E(B)-E(A)) is positive as well.. We have a diagram showing the potential energy changes for a reaction pathway.. Point A represents the potential energy of the reactants.
late 14c., "possible" (as opposed to actual), "capable of being or becoming," from Old French potenciel and directly from Medieval Latin potentialis "potential," from Latin potentia "power, might, force;" figuratively "political power, authority, influence," from potens "powerful," from potis "powerful, able, capable; possible;" of persons, "better, preferable; chief, principal; strongest, foremost," from PIE root *poti- "powerful; lord." The noun, meaning "that which is possible, anything that may be" is attested by 1817 (Coleridge), from the adjective. Middle English had potencies (plural) "a caustic medicine" (early 15c.).
Draw the energy profile diagram and also calculate the activation energy of the forward reaction for a reaction . Energy released is equal to twice the activation energy of the forward reaction and of the backward reaction is 120 kJ/mole.
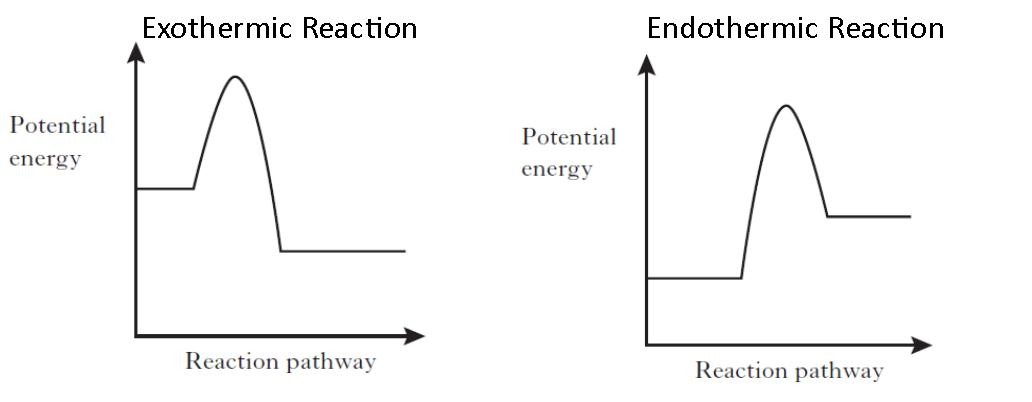
From the above diagram, the exothermic reactions show the potential energy of the product lower than that of the potential energy of the reactant whereas endothermic reactions show the potential energy of the product higher than that of the potential energy of the reactant.
In this reaction, the total energy of the reactants is 80 kJ mol-1, the total energy of the products is -90 kJmol-1 and the activation energy for the forward reaction is 120 kJ mol-1. a) Draw a diagram of the energy profile for this reaction. Label the diagram. b) State whether the reaction is endothermic or exothermic.
The potential energy diagram for a reaction starts at 180 kJ and ends at 300 kJ. What type of reaction does the diagram best represent? (5 points) Exothermic reaction Endothermic reaction Reaction between two solids Reaction between two liquids. Categories English. Leave a Reply Cancel reply.
Potential Energy Diagram Worksheet. Adrianne Lefevre. November 15, 2021. Potential Energy Diagram Practice Endothermic And Exothermic Reactions Potential Energy Energy Activities Exothermic Reaction. Chemistry 30 Chemical Kinetics Potential Energy Diagrams Revisited Chemistry Education Teaching Chemistry Chemistry.
The figure below shows basic potential energy diagrams for an endothermic. Reading Potential Energy Diagrams - YouTube (Jeremy Hansen) Note: the molar gibbs free energy of a pure element is often give the symbol µAo, as it is equivalent to the chemical potential of the pure element. Having presented an energy band diagram interpretation of our ...
Usually, potential energy diagrams were plotted using reaction pathway/reaction progress in the x-axis and potential energy in the y-axis. For exothermic reactions, the total energy of the products will be leass than the reactants. Since, energy is released during the reaction process. On the other hand, for an endothermic reaction the energy ...
1550s, "be taken or regarded as," also "be in favor of," from go (v.) + for (adv.). Meaning "attack, assail" is from 1880. Go for broke is from 1951, American English colloquial.
"action in resistance or response to another action or power," 1640s, from re- "back, again, anew" + action (q.v.). Modeled on French réaction, older Italian reattione, from Medieval Latin reactionem (nominative reactio), a noun of action formed in Late Latin from the past-participle stem of Latin reagere "react," from re- "back" + agere "to do, perform." Originally a word in physics and dynamics. In chemistry, "mutual or reciprocal action of chemical agents upon each other," by 1836. The general sense of "action or feeling in response" (to a statement, event, etc.) is recorded from 1914. Reaction time, "time elapsing between the action of an external stimulus and the giving of a signal in reply," attested by 1874.
The diagram shows the potential energy changes for a reaction pathway. Part 1: Does the diagram illustrate an endothermic or an exothermic reaction? Give reasons in support of your answer. Part 2: Describe how you can determine the total change in enthalpy and activation energy from the diagram and if each is positive or negative.
Mar 23, 2018 · reaction? __f__ 6. Is the reaction exothermic or endothermic? _endo_ 7. Which letter indicates the activation energy of the reverse reaction? ___d___ 8. Which letter indicates the heat of reaction of the reverse reaction? ___f___ 9. Is the reverse reaction exothermic or endothermic? __exo__ 1. The PE of the reactants of the forward reaction is
Hey All, I'm new to the forum here and a struggling O-Chem student. I'm taking OChem 1, and although I do enjoy it, I'm still timid about my answers. I'm doing a test review problems, and I was wondering if I could get some help to verify my answers. I'm not hundred percent sure, and any inputs would be greatly appreciated. Anything that isn't clear, too vague, etc. Thank you in advance! :) (**4**). Explain why free radical halogenation produces racemic mixtures of products. -Because the ra...
prefix usually meaning "away, opposite, completely," from Old English for-, indicating loss or destruction, but in other cases completion, and used as well with intensive or pejorative force, from Proto-Germanic *fur "before, in" (source also of Old Norse for-, Swedish för-, Dutch ver-, Old High German fir-, German ver-); from PIE *pr-, from root *per- (1) "forward," hence "in front of, before, toward, near, against." In verbs the prefix denotes (a) intensive or completive action or process, or (b) action that miscarries, turns out for the worse, results in failure, or produces adverse or opposite results. In many verbs the prefix exhibits both meanings, and the verbs frequently have secondary and figurative meanings or are synonymous with the simplex. [Middle English Compendium] Probably originally in Germanic with a sense of "forward, forth," but it spun out complex sense developments in the historical languages. Disused as a word-forming element in Modern English. Ultimately from the same root as fore (adv
Exothermic Reactions. A chemical reaction that releases energy (as heat) is called an exothermic reaction. This type of reaction can be represented by a general chemical equation: Reactants → Products + Heat. In addition to methane burning, another example of an exothermic reaction is chlorine combining with sodium to form table salt.
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ...
1590s, "force of expression," from French énergie (16c.), from Late Latin energia, from Greek energeia "activity, action, operation," from energos "active, working," from en "at" (see en- (2)) + -ergos "that works," from ergon "work, that which is wrought; business; action" (from PIE root *werg- "to do"). Used by Aristotle with a sense of "actuality, reality, existence" (opposed to "potential") but this was misunderstood in Late Latin and afterward as "force of expression," as the power which calls up realistic mental pictures. Broader meaning of "power" in English is first recorded 1660s. Scientific use is from 1807. Energy crisis first attested 1970.
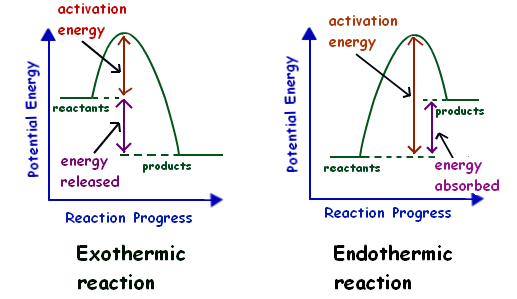
Energy profiles or energy diagrams for endothermic and exothermic reactions with or without a catalyst tutorial with worked examples for Chemistry students. An exothermic reaction is one in which heat energy is given out. The difference between the potential energies of products and reactants gives the heat of reaction.
This chemistry video tutorial focuses on potential energy diagram s for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... The potential energy diagram and balanced equation shown below represent a reaction between solid carbon and hydrogen gas to produce 1 mole of C2H4(g) at 101.3 kPa and 298 K. Identify one change in the reaction conditions, other than ...
For the above Engothermic Potential Energy Diagram: 1. Forward Activation Energy is 250kJ-50kJ = 200kJ. 2. Potential Energy of the Products = 100kJ. 3. Potential Energy of Reactants = 50kJ. 4. Forward Heat of Reaction = 100kJ - 50kJ = 50kJ (Notice the result is positive) Know the different parts of both the Exothermic and Endothermic Potential ...
Old English for "before, in the sight of, in the presence of; as far as; during, before; on account of, for the sake of; in place of, instead of," from Proto-Germanic *fur "before; in" (source also of Old Saxon furi "before," Old Frisian for, Middle Dutch vore, Dutch voor "for, before;" German für "for;" Danish for "for," før "before;" Gothic faur "for," faura "before"), from PIE root *per- (1) "forward," hence "in front of, before," etc. From late Old English as "in favor of." For and fore differentiated gradually in Middle English. For alone as a conjunction, "because, since, for the reason that; in order that" is from late Old English, probably a shortening of common Old English phrases such as for þon þy "therefore," literally "for the (reason) that."
How To Read Potential Energy Diagrams . Endothermic Reaction Lesson Plans Worksheets Lesson Planet . ... Potential Energy Diagrams . Potential Energy Diagrams Ck 12 Foundation . Chemistry 12 . Potential Energy Diagrams Worksheet With Answers Download Printable Pdf Templateroller . Post Navigation. Previous Article Adding Decimals Worksheets Pdf.























0 Response to "43 potential energy diagram for endothermic reaction"
Post a Comment