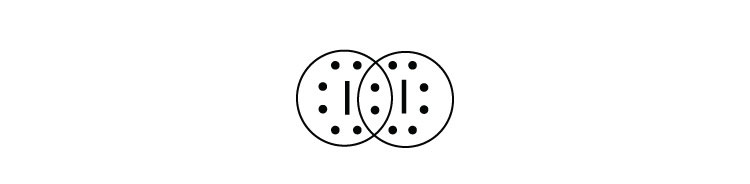
43 lewis dot diagram for iodine
The lewis dot structure ignores the nucleus and all non-valence electrons, displaying only the valence electrons of an atom. If the atom is a noble-gas atom, two alternative procedures are possible. Either we can consider the atom to have zero valence electrons or we can regard the outermost filled shell as the valence shell. In the Lewis structure of ICl4- there are total of 36 valence electrons. Since Iodine (I) is below Period 3 on the periodic table it can hold more than 8 electrons. In the Lewis structure for ICl4- the Iodine atom has 12 valence electrons.
Draw a Lewis dot diagram for an iodine molecule. Draw a Lewis dot diagram for a hydrogen molecule. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. In your own words, describe...

Lewis dot diagram for iodine
Bromine has atomic number 35, which means it has 7 electrons in its valence shell. On the other hand, iodine is located in group 17 (main group 7), which means it has 7 valence electrons. Valence electrons can be counted using a Lewis electron dot diagram. … Hence, the Lewis structure of iodine trichloride would be: We can observe that every chlorine atom is surrounded by eight electrons but a central atom, iodine, is surrounded by ten electrons. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons ... 1 answerHint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, ...
Lewis dot diagram for iodine. Chemistry worksheet lewis dot structures covalent compounds answer key. Lithium oxygen neon magnesium iodine boron sulfur carbon phosphorus ii. You should consult the lewis structure rules and a periodic table while doing this exercise. Write the electron dot structure lewis dot structure for covalent compounds or ions. 6 Nonzero formal charges are indicated for each atom in the structure once the total number of electrons is correct. Schiferl D, Review of Scientific Instruments, 1977, 48, 24. The Lewis acidity of bismuth(III) halides: a DFT analysis. STUDY. 5. Created by. Both molecules are pyramidal (C3v symmetry), and their geometries are characterized by the following bond lengths (rg) and bond angles ... Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you'll need to put a total of 12 valence electrons on the Iodine atom in order to draw the Lewis structure. Exercise 3.5. 1. Use Lewis electron dot diagrams to illustrate the covalent bond formation in Cl 2. Answer. More than two atoms can participate in covalent bonding, although any given covalent bond will be between two atoms only. Consider H and O atoms: The H and O atoms can share an electron to form a covalent bond:
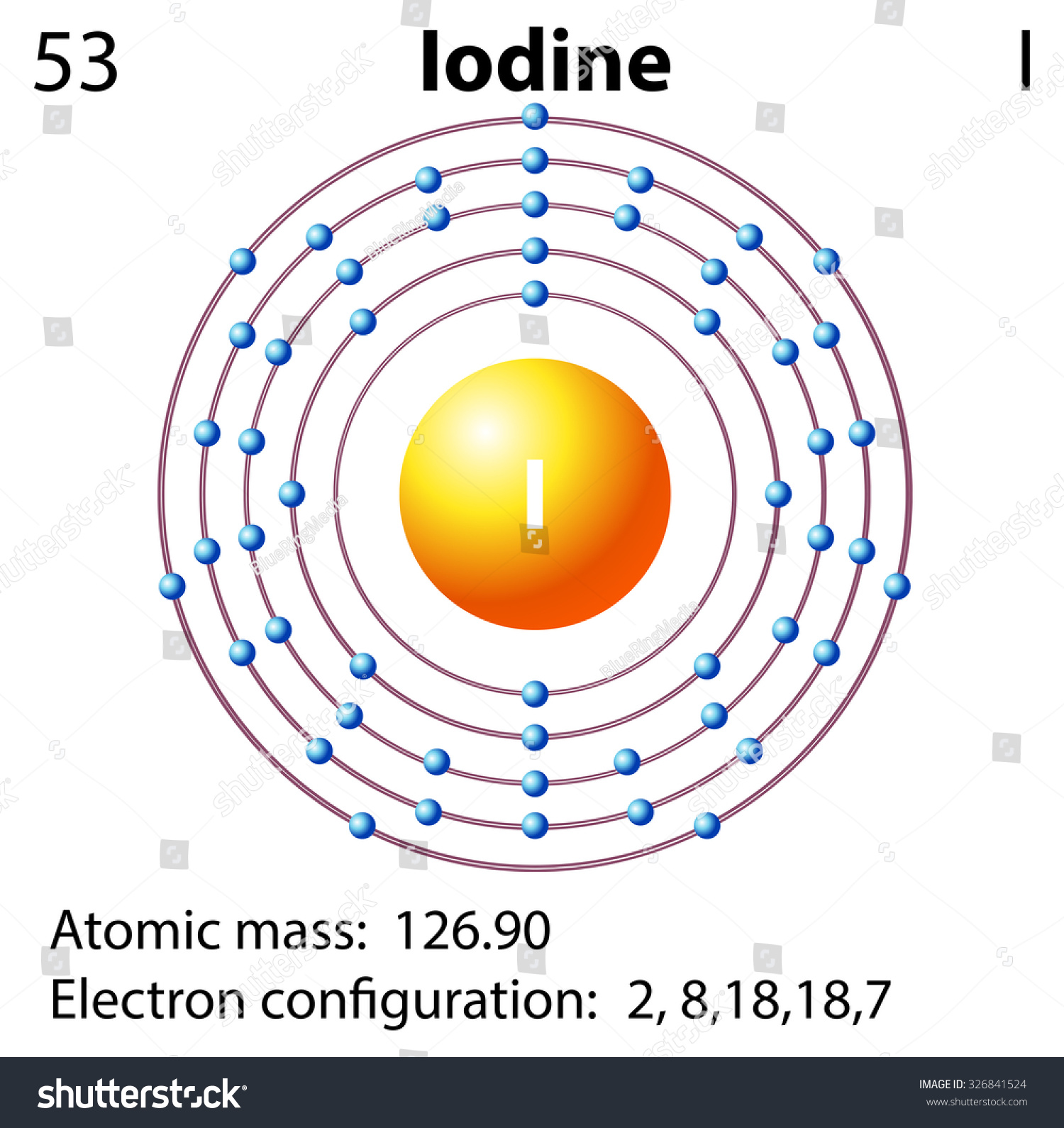
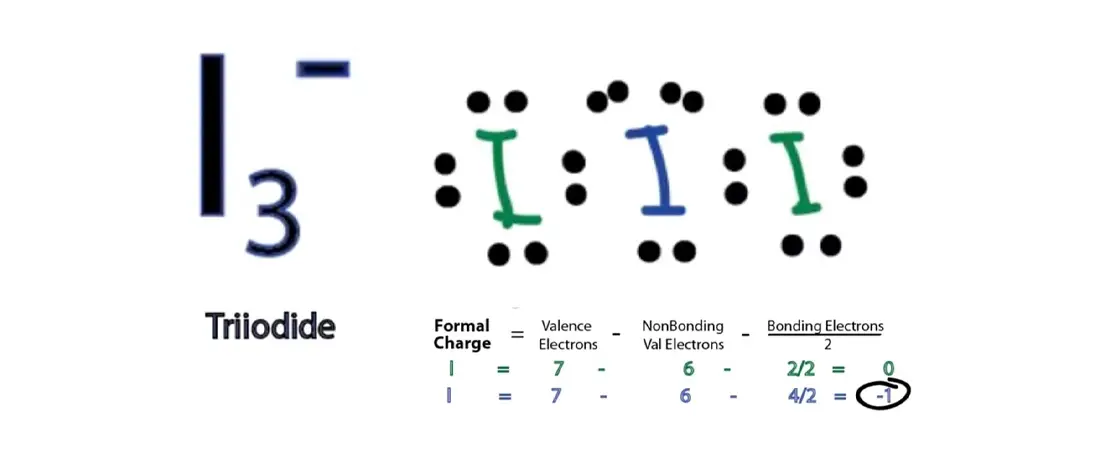
The formation of a water molecule from two hydrogen atoms and an oxygen atom can be illustrated using Lewis dot symbols (shown below). The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding ... Atomic Structure of Iodine ... Electron Configuration: 1s2 2s2p6 3s2p6d10 4s2p6 ... Forming the solitary bonds with the other two iodine, we find out that there room 3 lone pairs and 2 bond pairs because that the main iodine. 5. Check the formal charge, we placed the an unfavorable charge external as every the diagram above.Thus, lewis structure is done. Hybridization of I3. The hybridization of I3 (Triiodide ion) is sp3d. Note That The Overall Charge On This Ion Is -1. (a) The structure actually represents an ion. The IF_4""^ (-) has a total number of 36 valence electrons: 7 from iodine, 7 from each of the four fluorine atoms, and 1 from the negative charge. Answer Save. Formal charges are easy enough, Formal charge = # of valence elctrons - (# non-bonding ...
Lewis dot structure worksheet 1. So its a nice tool to explore how atoms bond into more complex substances. CH 4 CCl 4 CO 2 N 2 BeCl 2 BF 3 C 2H 4 C 2H 6 CO O 2 NO NO 2-NO 3-NH 3 NH 4 O 3 ClF3 SO 4 SF 6 SF 4 I 3-XeCl 2 PF 5 CO 3 BrF 5. No matter what style of writing you wish to master it is critical that you understand the terms and grammar ... Electron Dot Structures Teachers Answer Key. Worksheet Lewis Dot Structure Worksheet Answers electron dot diagrams and lewis structures worksheet answers 9th higher. Electron point diagram and worksheet structure lewis. Worksheet Electron Dot Diagrams and Lewis Structures Responses Lobo Magnesium iodine boro sulphur carbon phosphorus ii. Apr 28, 2021 — Iodine has seven valence electrons, because it is in group seven on the periodic table and is a halogen. The dot structure would be the element ... Iodine element atom structure and properties including diagram and all information for chemistry science education. They produced it by bombarding uranium-238 with deuterium nuclei alpha particles . Iodine forms compounds with many elements, but it is less reactive than the other. Chemical Elements 83 Diagram, Quizlet.
In lewis diagrams, valence electrons are represented by dots. So when we draw the Lewis diagram for the Cl atom we draw 7 dots around it. Now we move on to the steps of drawing a Lewis diagram:-1. The first step is to count all the valence electrons of each molecule. In the case of IF5, The Iodine atom has 7 valence electrons.
When two atoms share a single pair of electrons, the bond is called a single covalent bond, or simply, a single bond . When writing out a Lewis structure, a dash is used to represent a shared pair of electrons in place of two dots. Therefore, the Lewis structure for iodine monobromide, IBr, is shown in Figure 11.4. 1:
Iodine is below Period Two on the periodic table so it can have an expanded octet (hold more than eight valence electrons). In the Lewis structure for IF5 you' ...Oct 25, 2016 · Uploaded by Wayne Breslyn
Draw a Lewis dot diagram for an iodine molecule. Draw a Lewis dot diagram for a hydrogen molecule. True or false: When drawing a covalent bond, it is proper to use a line connect atoms and a line represents 2 electrons. In your own words, describe the difference between a bond that is nonpolar covalent and polar covalent.
A Lewis electron dot diagram (or electron dot diagram) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
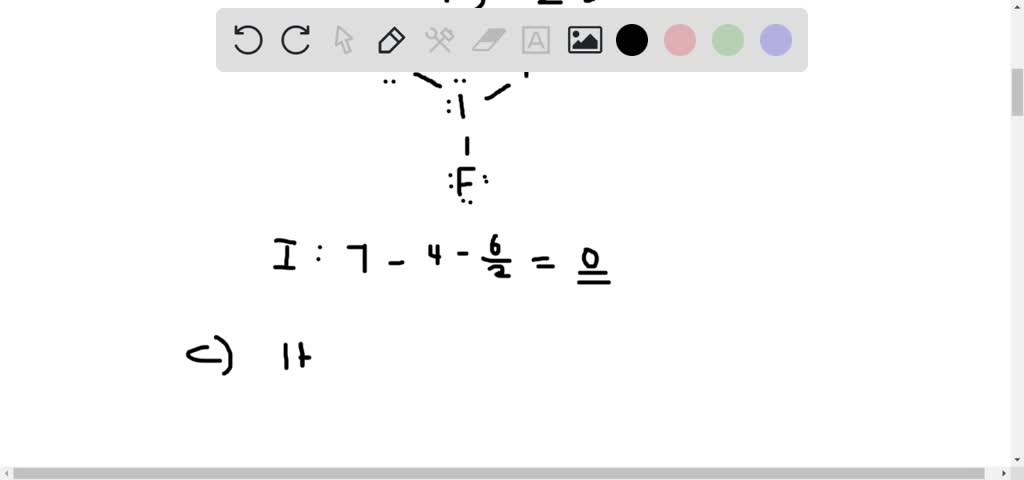
IF3 Lewis Structure, Hybridization, Molecular Geometry, and Polarity. IF3 or Iodine trifluoride is a yellow solid and is categorized as an interhalogen compound. It is highly unstable and decomposes above the temperature of -28 degrees Celsius. The molar mass of IF3 is 183.9 g/mol. IF3 can be prepared using two methods:-.
The resulting lewis electron dot structure is: Lesen Sie auch: 10+ 5 Relay Stabilizer Circuit. Source: d2vlcm61l7u1fs.cloudfront.net. Fluorine is the most electronegative element, and so therefore, for silicon tetrafluoride, we're going to put the silicon atom at the center of our dot structure, since it is the least electronegative of those two.
As we know that iodine has '7' valence electrons and oxygen has '6' valence electrons. Therefore, the total number of valence electrons in = 7 + 3(6) + 1 = 26. According to Lewis-dot structure, there are 10 number of bonding electrons and 16 number of non-bonding electrons. Now we have to determine the formal charge on the central iodine atom.
Lewis dot structures help predict molecular geometry. This example problem shows the steps to draw a structure where an atom violates the octet rule.. Molecular Geometry T Shaped . A Lewis Structure is a representation of covalent molecules or polyatomic ions where all the valence electrons are shown .... ICl3 c. TeF4 d. PCl5 122.
The lewis structure of ICl5 can be made by following the following steps: Step 1: Calculate the total valence electrons in the compound. For the first step, it is required to know the electronic configuration of iodine and chlorine. The electronic configuration of iodine is [Kr]4d105s25p5.
40 2005 toyota corolla wiring diagram pdf; 42 lewis dot diagram for iodine; 42 small business network setup diagram; 38 2003 silverado wiring diagram; 37 this diagram of the human life cycle shows that... 38 a design class diagram is also called a data mo... 40 whirlpool duet washer diagram; 37 ford 8n throttle linkage diagram
1 answerHint: The electron dot diagram is the diagram which clearly shows the bonding between the atoms of a molecule along with the lone pairs of electrons, ...
Hence, the Lewis structure of iodine trichloride would be: We can observe that every chlorine atom is surrounded by eight electrons but a central atom, iodine, is surrounded by ten electrons. It is one of the exceptions of the octet rule, i.e., the elements of the third period or beyond the third period of the periodic table have 3d electrons ...
Bromine has atomic number 35, which means it has 7 electrons in its valence shell. On the other hand, iodine is located in group 17 (main group 7), which means it has 7 valence electrons. Valence electrons can be counted using a Lewis electron dot diagram. …








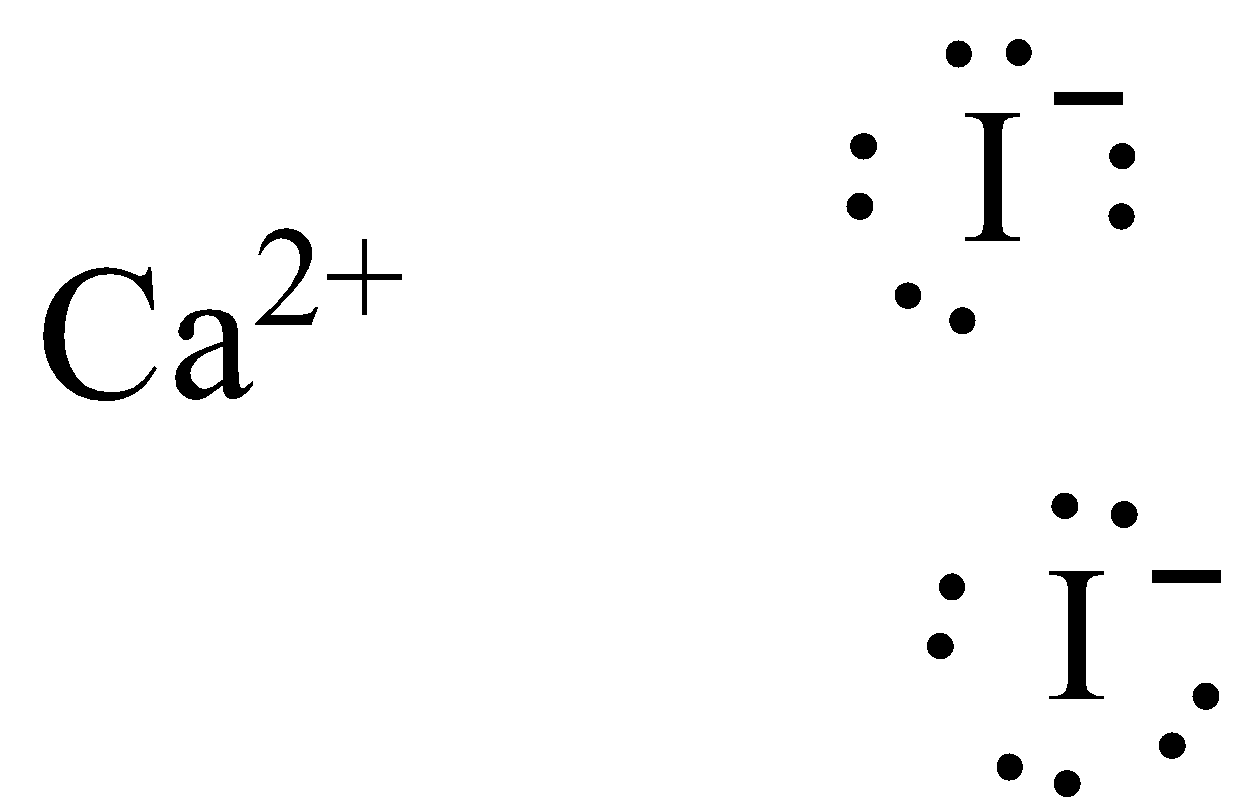



/ICl3_LD-56a12a2b3df78cf77268034c.png)











0 Response to "43 lewis dot diagram for iodine"
Post a Comment