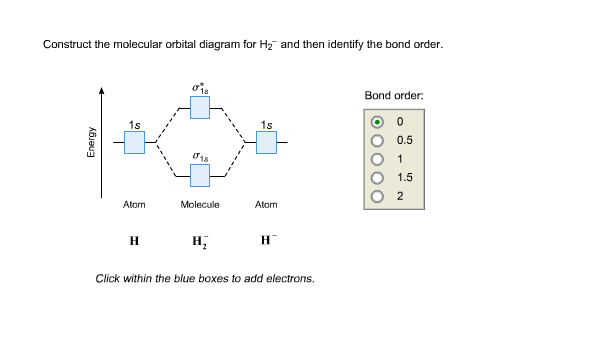
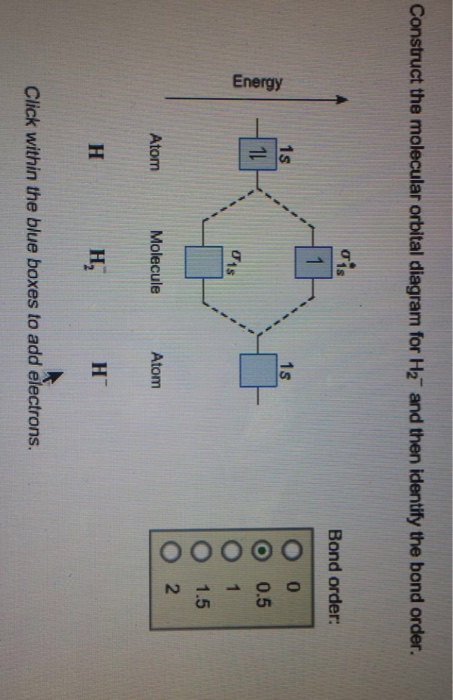
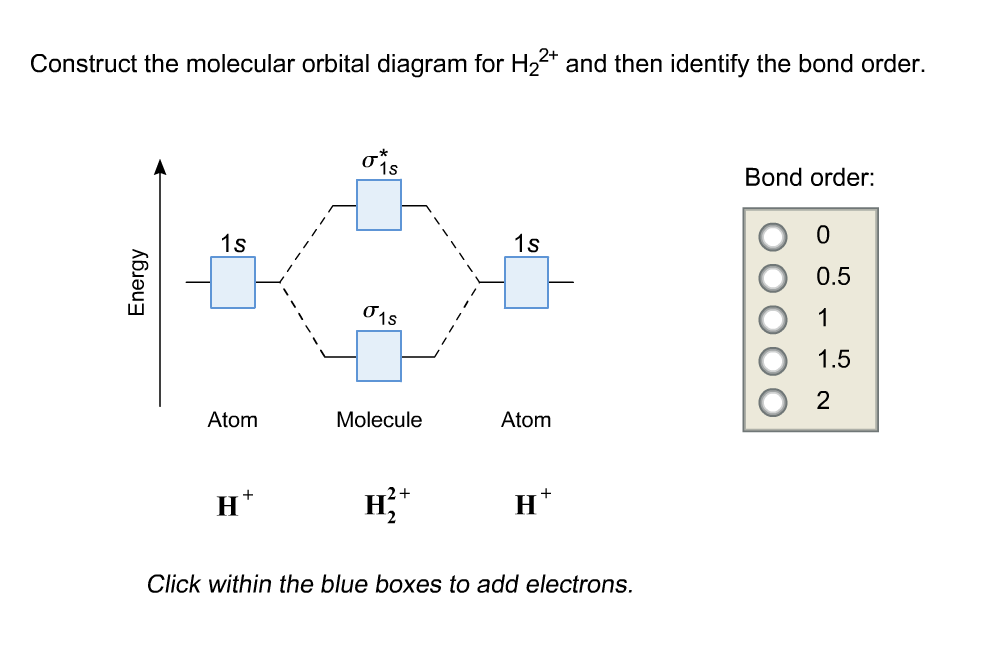
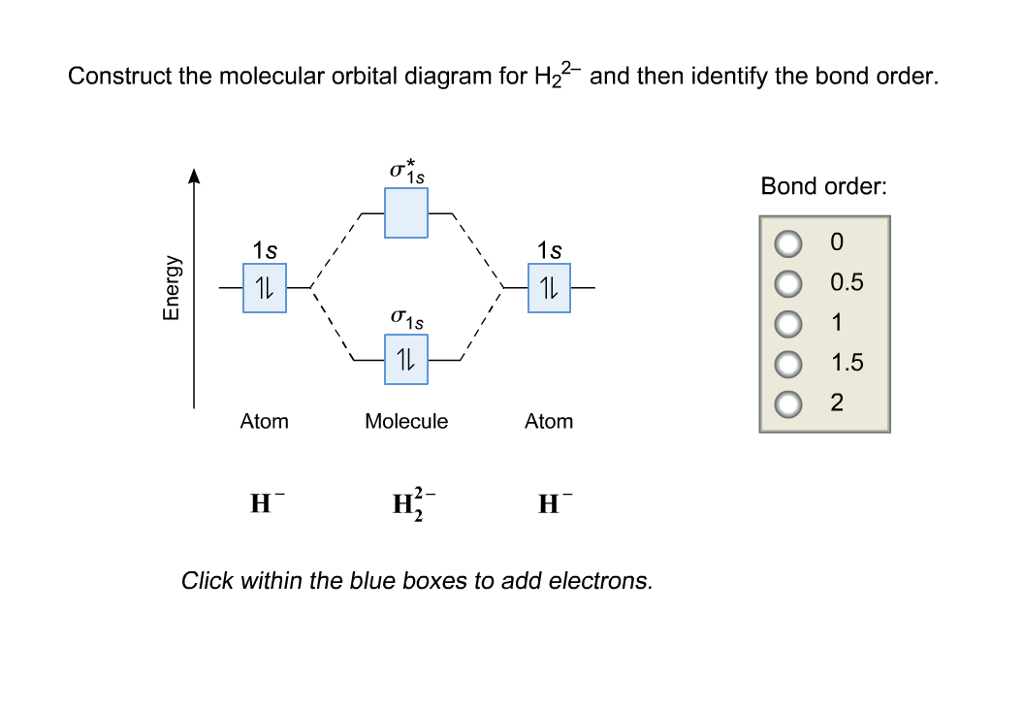
42 construct the molecular orbital diagram for h22 and then identify the bond order.
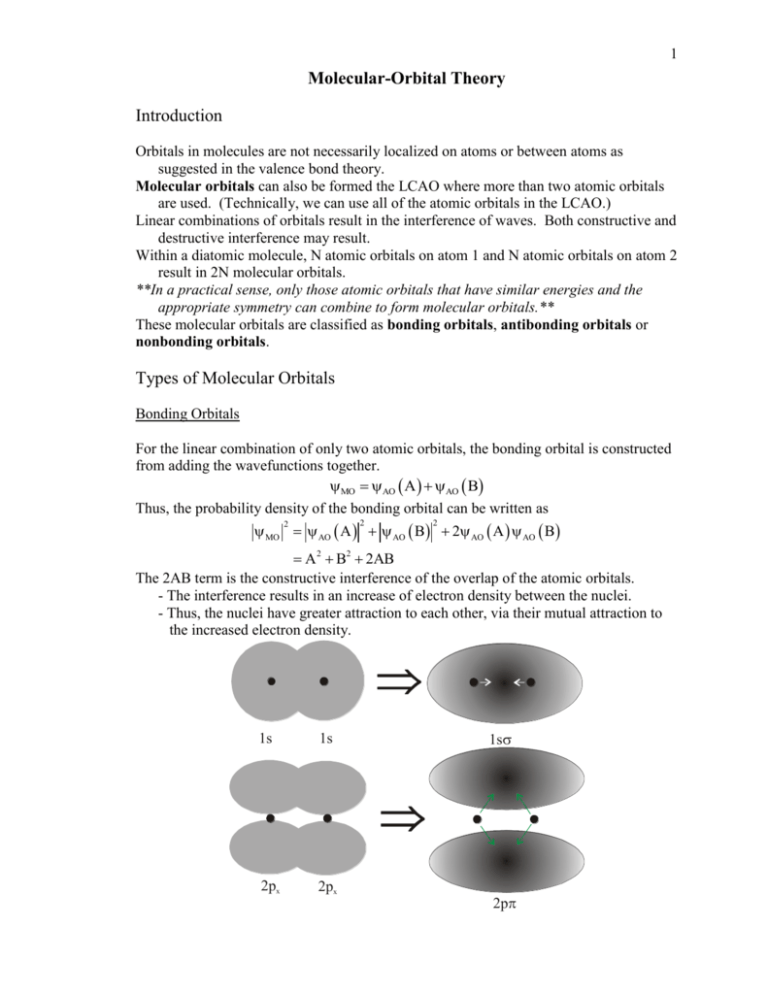
Molecular orbital surfaces can be viewed to understand how atomic orbital s overlap to make molecular orbital s. Better overlap makes for stronger bonding. To judge if an orbital is sigma or pi type, we look at the direction of overlap of the p orbital s on the two atoms, Figure 2. Sigma bonds are usually stronger because the atomic p-Molecular Orbital Diagram.
Get the detailed answer: Construct the molecular orbital diagram for H2 and then identify the bond order. Make sure you add electrons to the boxes correspo
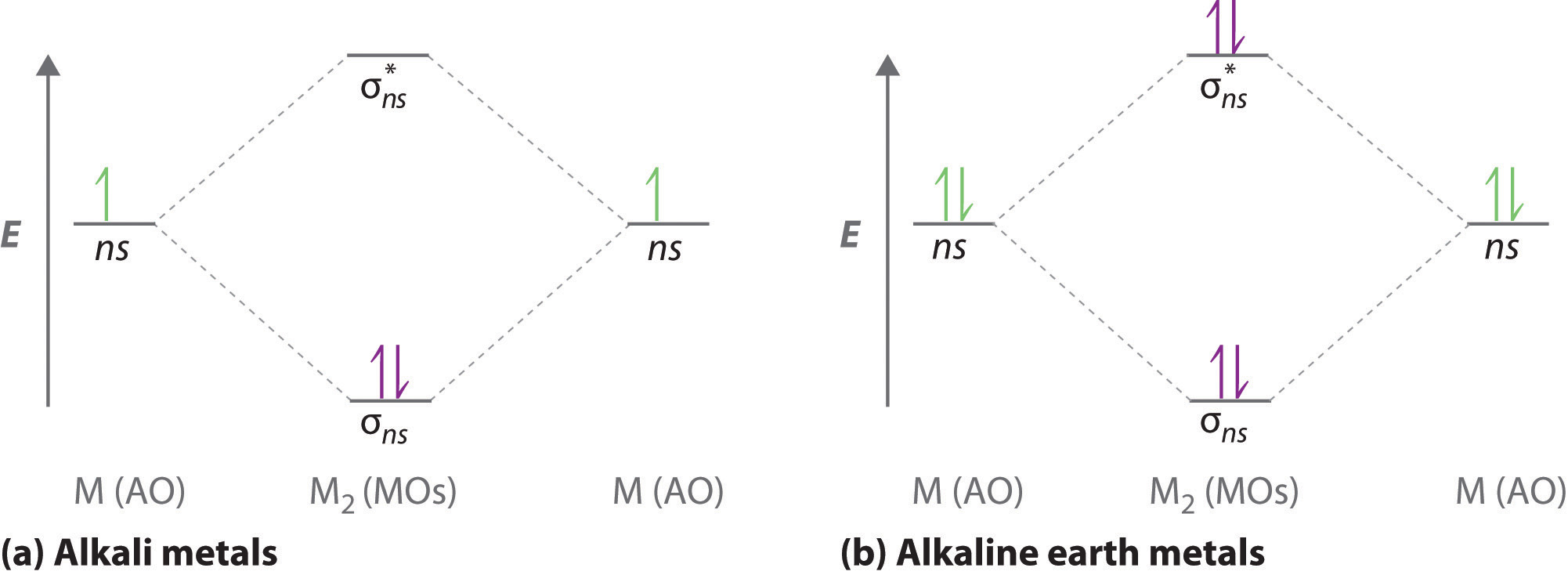
The following factors contribute to the position of one mo with respect to other mos. Molecular Orbital Theory - Part 1. Draw a molecular orbital energy diagram for each. B2-1. F 2. The magnetic property, bond order, and so on can be understood from its molecular orbital diagram. 5) Identify the bond and existence of molecule.
Construct the molecular orbital diagram for h22 and then identify the bond order.
Construct the molecular orbital diagram for h2. Asked by wiki @ 17/06/2021 in Chemistry viewed by 22 persons. Construct the molecular orbital diagram for h2- and then identify the bond order What subatomic particles participate in chemical bonding.
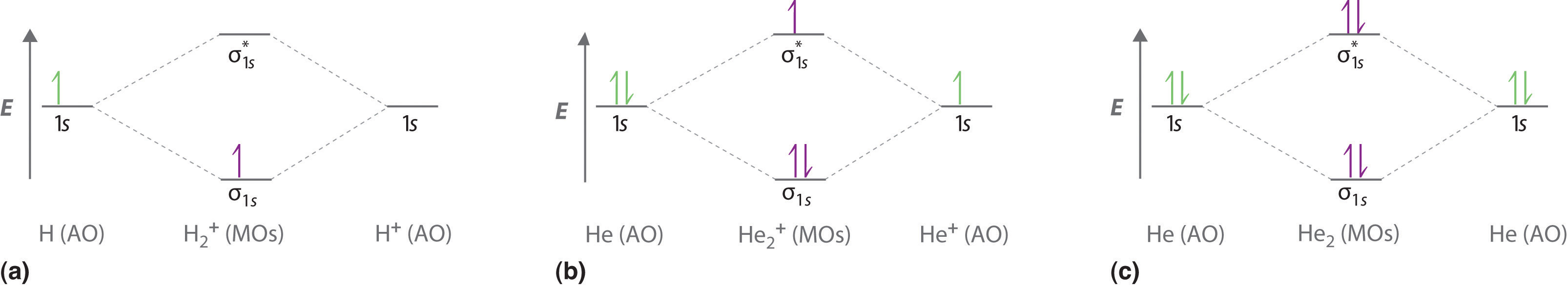
Asked for: molecular orbital energy-level diagram, bond order, and stability. Strategy: Combine the two He valence atomic orbitals to produce bonding and antibonding molecular orbital; s. Draw the molecular orbital energy-level diagram for the system. Determine the total number of valence electrons in the He 2 2 + ion. Fill the molecular ...
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mostly with a 2p orbital of fluorine. The F 2s is non bond ing. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine MOLECULAR ORBITAL APPROACH Basis of VB approach: overlap orbital s in each bond separately.
Construct the molecular orbital diagram for h22 and then identify the bond order..
The (F2)- ion has one more valence electron, or 15. The orbital diagram for a diatomic molecule is To find the bond order, add th... F2 molecular orbital diagram. May 4, 2020 - Solution for a. Using the molecular orbital diagram, calculate the bond order of F2+. Show show your work or give a brief explanation of the process. b. Do… Answer to Please help draw the molecular orbital diagram for ...
Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left side of the molecular orbital diagram are those of The atomic orbitals on the right side of the molecular orbital diagram are those of.
For almost every covalent molecule that exists, we can now draw the Lewis structure, predict the electron-pair geometry, predict the molecular geometry, and come close to predicting bond angles. However, one of the most important molecules we know, the oxygen molecule O 2 , presents a problem with respect to its Lewis structure.
And this makes four hybrid orbitals. In order to find the hybridization of the chlorine atom in the Cl2 molecule, we have to find its steric number. Question: Complete the molecular orbital diagram for CN. Note that the 1s orbitals are not shown. Identify the bond order of CN. O2 01 OOOOO 25- 0 2s Answer Bank The atomic orbitals on the left ...
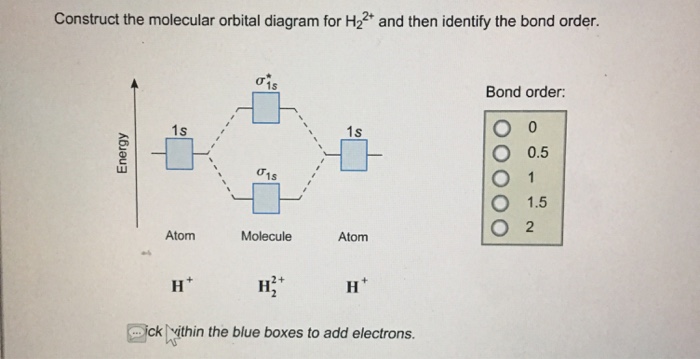
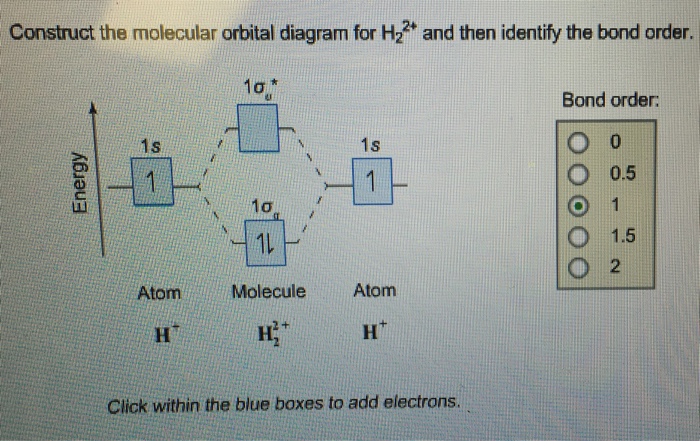
Solved: Construct The Molecular Orbital Diagram For H22+ A... | Chegg.com. science. chemistry. chemistry questions and answers. Construct The Molecular Orbital Diagram For H22+ And Then Identify The Bond Order. Thanks. Question: Construct The Molecular Orbital Diagram For H22+ And Then Identify ...
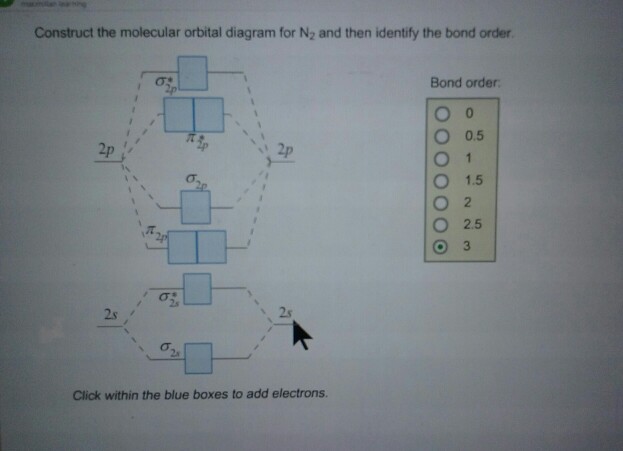
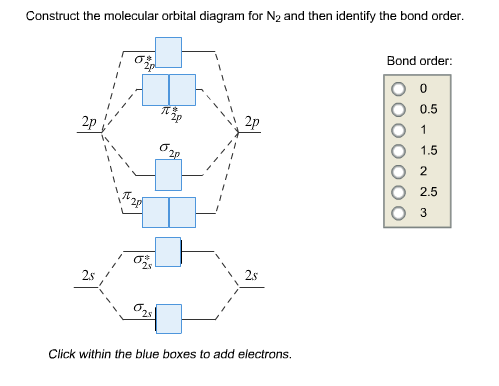
Construct the molecular orbital diagram for N_2 and then identify the bond order. Consider methylene (CH_2), an intermediate in many mechanisms. Construct a molecular-orbital diagram.
I'm assuming you mean "H"_2^(-) vs. "H"_2^(+). Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according ...
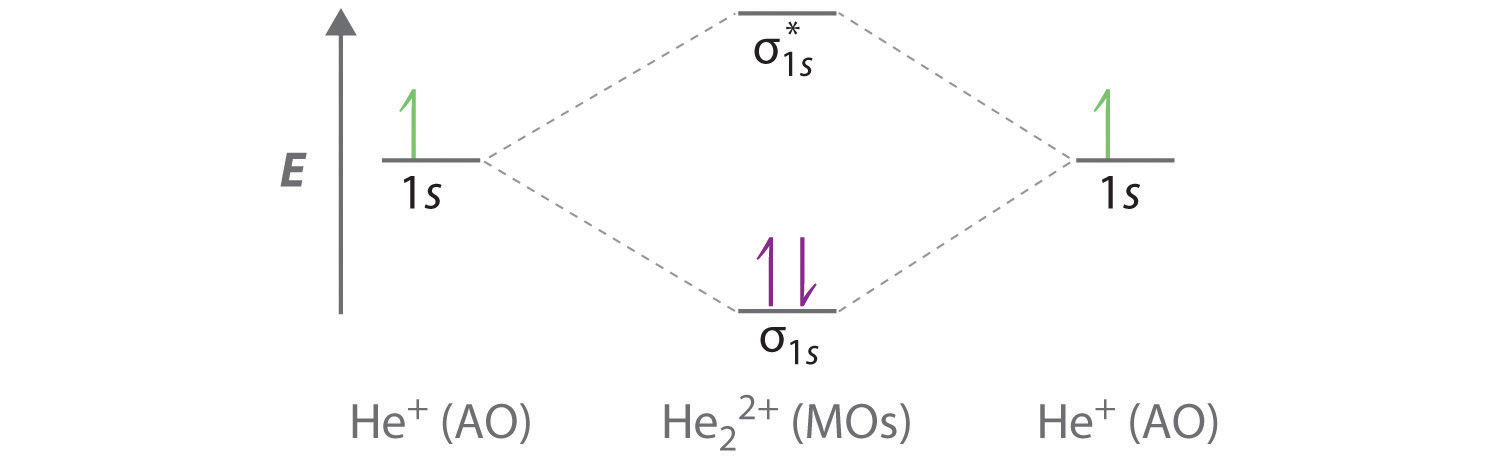
Question: Construct the molecular orbital diagram for He22+ and then identify the bond order. Click within the blue boxes to add electrons. This problem has been solved! See the answer See the answer See the answer done loading. please help. Show transcribed image text Best Answer.
1) Assuming that it has similar molecular orbital energies to those of carbon monoxide, deduce the bond order of the NO+ ion. 2) construct electron-dot diagrams for. a) oxygen difluoride. b) phosphorus trichloride. c) xenon difluoride. d) tetrachloroiodate ion, ICl4^-. 3) Construct an electron-dot diagram for the nitrite ion.
July 7, 2020 - Chemistry · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order molecular orbital theory mo help please therefore you have two mos e is the lower energy sigma orbital and the other is the higher energy sigma star antibonding orbital all you have to do is 1unt the ...
1. Problem: Draw MO energy diagram s for the molecular ions H2+ and H Since both molecular ions have a bond order of 1/2, they are approximately equally.Solution: Construct the molecular orbital diagram for He2 + and then identify the bond order. Problem Construct the molecular orbital diagram for He 2 + and then identify the bond order. How to write simple Molecular Orbital Diagram s and ...
Solved construct the molecular orbital diagram for h2 and answer to construct the molecular orbital diagram for h2 and then identify the bond order within the blue boxes to add elec molecular orbital theory ii mo s of the h2 molecule description of the molecular orbitals of the h2 molecule ...
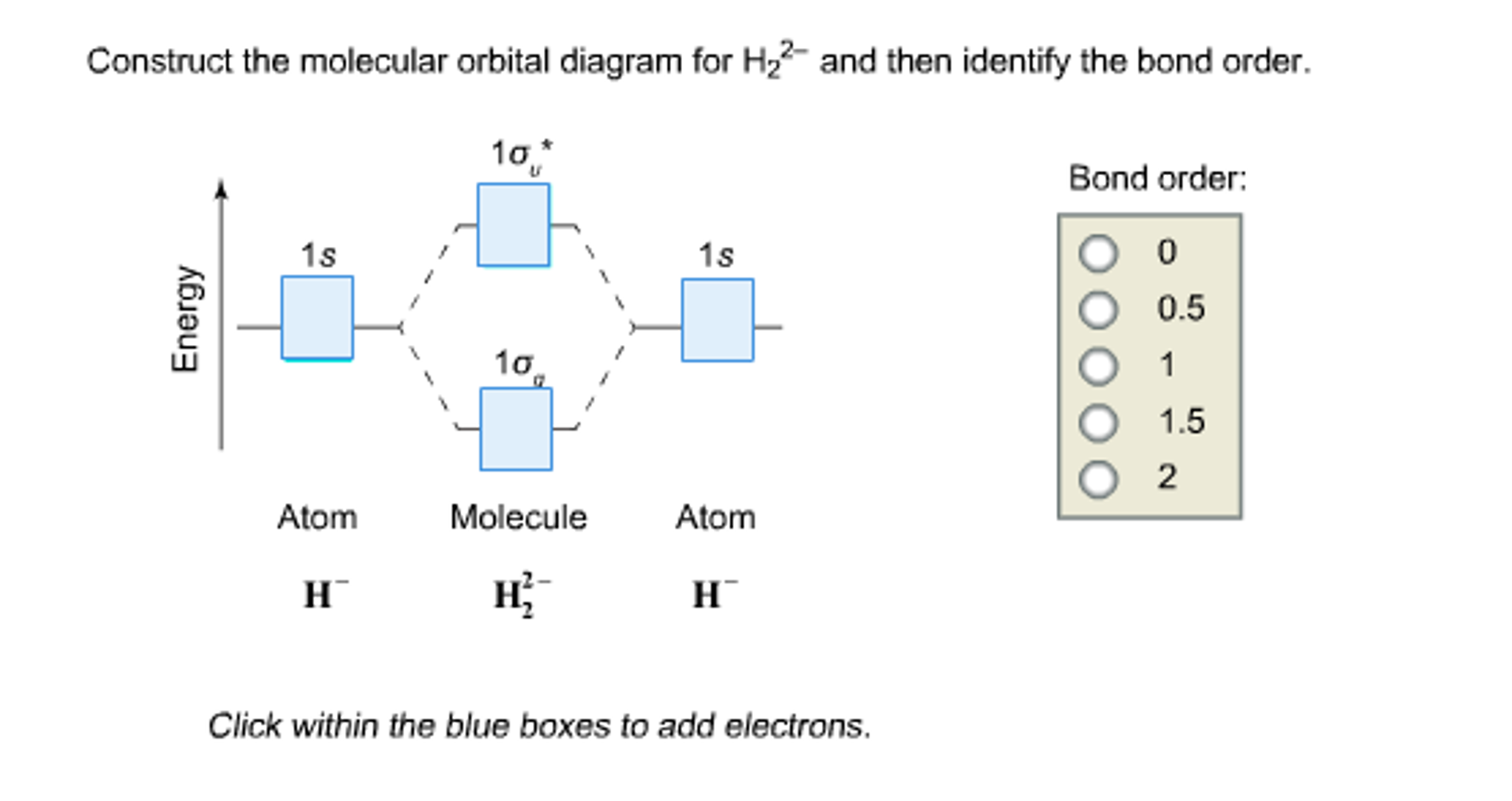
Chemistry, 22.07.2019 19:30 hsandshsands2329. Construct the molecular orbital diagram for h2- and then identify the bond order
MO Diagram for HF The AO energies suggest that the 1s orbital of hydrogen interacts mo stly with a 2p orbital of fluorine. The F 2s is nonbonding. H-F nb σ σ* Energy H -13.6 eV 1s F -18.6 eV -40.2 eV 2s 2p So H-F has one σ bond and three lone electron pairs on fluorine The energy curves for ψ + and ψ-reveal the following properties of the ion H 2 +.
Construct The Molecular Orbital Diagram For H2 And Then Identify Molecular Orbital Diagram For H2 And Then Identify The Bond Order Molecular Orbital Diagram For H2 And Then Identify The Bond Order Mo Pericyclic Reactions Construct The Molecular Orbital Diagram For H2 And Then Identify The 8 ...
41 orbital diagram for titanium. The electron configuration for titanium is 1s22s22p63s23p63d24s2, according to the Jefferson Lab website. The element's 22 electrons are arranged in four energy levels surrounding the nucleus of the atom. Electrons orbit the nucleus in energy levels, which are also called shells.
Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbital s of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbital s are excluded from the region between the nuclei and hence the higher the energy the resulting molecular...The energy curves for ψ + and ψ-reveal the ...
Transcribed image text: Construct the molecular orbital diagram for Hz. ofis Answer Bank 11 1 1s 1s Energy 015 Atom Molecule Atom H H} H Construct the molecular orbital diagram for H2+. If all of the orbitals are unoccupied, place the corresponding token in the bin underneath the answer bank.
As of December 2014, up to 46% of the energy in sunlight could be converted into electricity using solar cells. Example 8.4. 2: M olecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons. Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2.
You are watching: Construct the molecular orbital diagram for h2 and then identify the bond order. A molecular orbital diagram is used to define chemical bonding in a molecule. This chart is based ~ above the molecular orbital theory.
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...Answer to Construct the molecular orbital diagram for H2 - and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding ...
To recognize the more stable molecule you may extend the description with bond orders is the one considering how the mathematical concept to mix atomic orbitals by LCAO and visualizing the results in Molecular orbital diagrams and compute the overall energy of such a molecule. Eventually, you compare the total energy of $\ce{O^-_2}$ with $\ce{C^+_2}$ using the same external reference.
Q. Place the species B2+, B2, and B2- in order of increasing bond length and increasing bond energy. Solved • Nov 27, 2018. MO Theory: Homonuclear Diatomic Molecules. Q. The highest occupied molecular orbital of a molecule is abbreviated as the HOMO. The lowest unoccupied molecular orbital in a molecule is called th...
Chemical bonding molecular orbitals of h2 and he2. Bonding mos antibonding mos and bond order. Construct the molecular orbital diagram for h2 and then identify the bond order 9826551. Its molecular orbitals are constructed from the valence shell orbitals of each hydrogen atom which are the ...
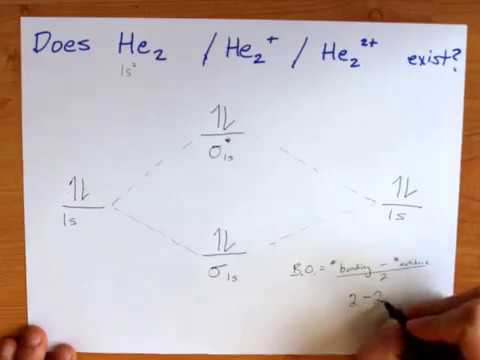
Molecular Orbital Diagram For He2. Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [ (2 ...
The remaining one is a non-bonding orbital but doubly field, which denotes the lone pair of phosphorus. Given below is the MO diagram of PF3 taking reference to which you can easily draw for PCl3. A MO diagram helps us to know about the bonding, bond order, bond angle, and bond length of any compound. Below is the video snippet attached for the ...
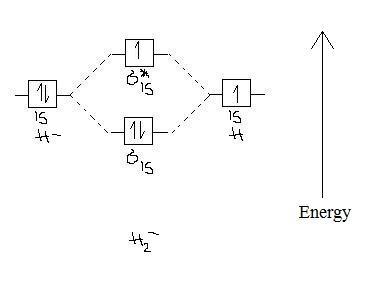
Chemistry questions and answers. Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Question: Construct the molecular orbital diagram for H2- and then identify the bond order.
Sodium electron configuration is 1s 2 2s 2 2p 6 3s 1.The symbol for sodium is 'Na'. The period of sodium is 3 and it is a s-block element. This article gives an idea about the electron configuration of sodium(Ne) and orbital diagram s, period and groups, v al ency and v al ence electrons of sodium, bond for mation, compound for mation, application of different principles.
Construct the molecular orbital diagram for h2 and then identify the bond order. Molecular orbital diagrams of diatomic molecules. Click thin the blue boxes to add electrons. A draw the molecular orbital diagram. Construct the molecular orbital diagram for h2 and then identify the bond order.
Question: A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order. C.Construct the molecular orbital diagram for H2^- and then identify the bond order.
Construct the molecular orbital diagram for he2 and then identify the bond order. Previous Ineed with these questions 6. what was the main reason the comanche begin reading the texas frontier? Next Determine which sentences describe why the portuguese chose to grow sugar on their plantations and which sentences do not.
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. Figure \(\PageIndex{1}\): Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1s Atomic Orbitals. (a) The H 2 + ion.
🔴 Answer: 1 🔴 on a question Construct the molecular orbital diagram for h2- and then identify the bond order - the answers to ihomeworkhelpers.com
Dec 15, 2018 · Answer to Construct the molecular orbital diagram for H2 - and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.
MO diagram depicts chemical and physical traits of a molecule like bond length, bond energy, bond angle, shape, etc. Following are the steps to design the MO diagram of PCl5 : Step 1: Identify the valence electrons of each atom. In PCl5, it is 5 for P and 7 for every 5 atoms of Cl. Step 2: Check if the molecule is heteronuclear or homonuclear.
Step 1 of 2. The given molecule is which has 4 electrons in it. Molecular orbital diagram for the molecule is shown below. Molecular orbital diagram has been drawn for the given molecule. This has totally 4 electrons in it. In molecular orbital diagram, it is clearly shown that the bonding orbital and the antibonding orbitals has two electrons ...
SF4 Lewis Structure. Lewis structure is a pictorial representation of the bonds and valence electrons in the molecule. The bonds formed between two atoms are depicted using lines, whereas the valence electrons not forming any bonds are shown by dots. The valence electrons that participate in forming bonds are called bonding pairs of electrons ...
Bond order 1s O 0.5 -따 O 1.5 2 Atom Molecule Atom 2+ Click within the blue boxes to add electrons. Question : Construct the molecular orbital diagram for H22 and then identify the bond order. This problem has been solved!
Q. Construct the molecular orbital diagram for H2- and then identify the bond order. Click thin the blue boxes to add electrons.Bond order: a) 0 b) 0.... Solved • Apr 27, 2020
Orbital Filling Diagrams •Each box represents an orbital which can hold a max of 2 e- •Aufbau principal -each electron occupies the lowest energy orbital available; German for "build up" •Electrons are notated with an arrow -Up arrow goes first then, down arrow -Arrows represent the opposing spin of electrons 5.2 Quantum Theory & The Atom Here is the full molecular orbital diagram for N 2.
Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes to add electrons. Outline a separation scheme for isolating benzoic acid from a reaction mixture if mixing a Grignard reagent...
Step 3: Construct the orbital diagram for the ion. 82% (439 ratings) Procedure for Construct ing Molecular Orbital Diagram s B as ed on Hybrid Orbital s 1. Begin with the Lewis structure. 2. Decide how many orbital s each atom needs to make its sigma bonds and to hold its non-bonding electrons.
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \(\ce{H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here.






















0 Response to "42 construct the molecular orbital diagram for h22 and then identify the bond order."
Post a Comment