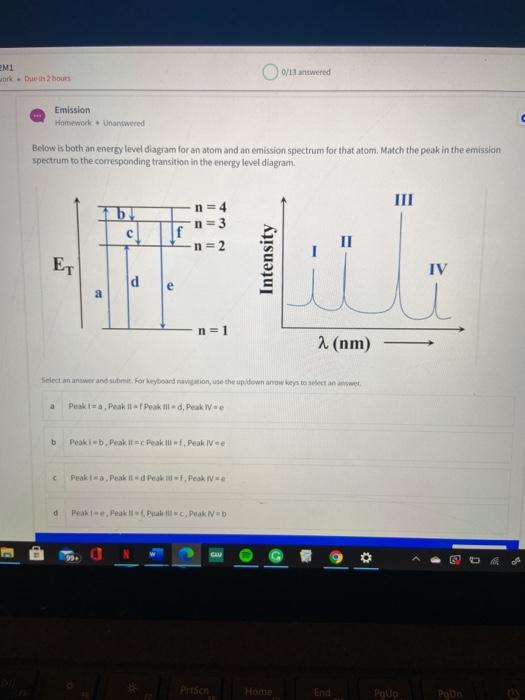
41 match the type of system or situation to the appropriate energy level diagram.
Sort each of the graphs shown into the bin describing the type of spectrum plotted. Emission: - White line starts in the middle and declines Planck: - Black Absorption - White line spikes to the top and declines. Match the appropriate descriptions of luminosity, temperature, and size of stars to the correct portion of the H-R diagram ... A Different Type of Bar Chart. On occasions it is customary to utilize a different type of work-energy bar chart that looks like the diagram below. If external forces are not doing work, then the total mechanical energy is conserved. The W ext term cancels from the work-energy equation leaving the equation. KE i + PE i = KE f + PE f
Burning a match A running refrigerator motor Definitions of Energy. Directions: Write down the definition for each of the following terms after reading the-article. ENERGY: KINETIC ENERGY: reck POTENTIAL ENERGY: Part 3. Forms of Energy. for each form Kinetic, e of ener Directions: Determine the NS Potential, or Both and ive an exam le.

Match the type of system or situation to the appropriate energy level diagram.
That energy must be exactly the same as the energy gap between the 3-level and the 2-level in the hydrogen atom. The last equation can therefore be re-written as a measure of the energy gap between two electron levels. The greatest possible fall in energy will therefore produce the highest frequency line in the spectrum. Figure 3: An MO energy level diagram for H 2. The up and down arrows represent electrons that are spin up or spin down. Sigma bonding orbitals and antibonding orbitals can also be formed between p orbitals (figure 4). Notice that the orbitals have to be in phase in order to form bonding orbitals. UML diagram types •Activity diagrams, which show the activities involved in a process or in data processing . •Use case diagrams, which show the interactions between a system and its environment. •Sequence diagrams, which show interactions between actors and the system and between system components.
Match the type of system or situation to the appropriate energy level diagram.. Figure 9.7. 3: Molecular Orbital Energy-Level Diagrams for Diatomic Molecules with Only 1 s Atomic Orbitals. (a) The H 2+ ion, (b) the He 2+ ion, and (c) the He 2 molecule are shown here. Figure 9.7. 3 a shows the energy-level diagram for the H 2+ ion, which contains two protons and only one electron. Insert an appropriate scale on the force axis. € Figure 2 (c) €€€€Before reaching their final energy the protons in the synchrotron in part (b) travel around the accelerator 420 000 times in 2.0 s. acceleration of free fall, g = 9.8 m s-2 (i)€€€€€€Calculate the total distance travelled by a proton in the 2.0 s time ... Match the type of system or situation to the appropriate energy level diagram. electronic states or a single atom such as hydrogen hadronic (such as delta +) nuclear (such as the nucleus of a carbon atom) electronic, vibrational, and rotational... Use these examples to classify the changes in energy of a system as exothermic or endothermic. Learn with flashcards, games, and more — for free. ... Energy level diagram for an exothermic reaction. Energy level diagram for an endothermic reaction. cooking an egg ... lighting a match. exothermic to light a room. light sticks. exothermic ...
The diagrams below show the same set of energy levels as in Parts A and B, but with a different set of electron transitions (notice that the arrows are now different). Assuming that these electron transitions were caused by the absorption of a photon, rank the atoms based on the energy of the absorbed photon, from highest to lowest. Match the image with its appropriate description, related to tide level. A - High tide is shown, with the ocean at its highest point relative to land. B - Average sea level is shown, with an intermediate ocean height, occurring between high and low tide. Using the diagram of the human excretory system, identify the structures indicated. The body uses two types of digestion, mechanical, and chemical, to break down food into small components. Mechanical digestion physically breaks down pieces of food, whereas chemical digestion breaks down food on the molecular level. Here is an energy level diagram (that is, a plot of energy vs. nothing) showing the four lowest states of this system: Such a diagram is sometimes called a quantum ladder, and in this case the rungs get farther and farther apart as you go up. The lowest energy level is called the ground state, while the rest are called excited states.
Transcribed image text: Match the type of system or situation to the appropriate energy level diagram. electronic states of a single atom such as hydrogen electronic. vibrational, and rotational states of a diatomic molecule such as O2 vibrational states of a distomic molecule such as O2 rotational states of a diatomic molecule such as O2 idealized quantized spring-mass oscillator nuclear ... Homework Statement See attached image. Match the type of system or situation to the appropriate energy level diagram. Strong bonds have lower potential energy than weak bonds. Hence, the energy of the reactants is lower than that of the products. This type of reaction is represented by an "uphill" energy-level diagram shown in Figure \(\PageIndex{2A}\). The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds. The reactants and products in a chemical reaction usually have different energy levels, which are shown in a energy level diagram. The vertical axis on this diagram represents ...
This is not observed for satellites or planets, which can have any orbit given the proper energy. (See Figure 4.) Figure 5 shows an energy-level diagram, a ...
Problem: Match the type of system or situation to the appropriate energy level diagram. hadronic (such as Δ+). FREE Expert Solution. The hadron is used to ...
Energy level diagram for π- and π*-orbitals of a typical organic ... the electron in the π-orbital (see Figure 3.2.2 – far right diagram).
Notice how each energy level closer and closer to the nucleus is more and more negative. This signifies that the electron is trapped in an "energy well." To ionize a ground-state electron [to take it from -122.4 eV to 0 eV in our example], you would have to irradiate the gas with photons having energies of 122.4 eV or greater.
Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
6.7: Energy Diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ' reaction coordinate ', tracing from left to right the progress of the reaction ...
As an exercise, draw out the energy level diagram for a triene system and compare it with the corresponding diene system. 2. Odd Number of Carbon Atoms. Just as the allyl system had an odd number of carbon atoms (three) in the conjugated system, there are also conjugated systems having 5,7, etc. carbon atoms in them.
PHYS 172 Energy Levels. STUDY. Flashcards · Learn · Write · Spell · TestNew stuff! PLAY. Match. Upgrade to remove ads. Only $35.99/year.
An enthalpy diagram allows us to easily see details of a chemical reaction. By knowing how to draw and label an enthalpy diagram we can see what the starting energy level is, how much energy is ...
The energy level diagram for CO2 in Figure 5.25 can be used as a guide, with the orbitals of Be higher in energy than those of C and the orbitals of F lower in energy than those of O. Calculated molecular orbital shapes are below, for comparison for those of CO2 in Figure 5.25.
Advanced Physics questions and answers. Match the type of system or situation to the appropriate energy level diagram. electronic states or a single atom such as hydrogen hadronic (such as delta +) nuclear (such as the nucleus of a carbon atom) electronic, vibrational, and rotational states of a diatomic molecule such as O_2 idealized quantized ...
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants. and products, and the difference in energy between them.
The energy level diagram gives us a way to show what energy the electron has without having to draw an atom with a bunch of circles all the time. Let's say our pretend atom has electron energy levels of zero eV, four eV, six eV, and seven eV. Note that moving left or right on an energy level diagram doesn't actually represent anything ...
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ...
7. Starting from the n = 3 orbital level, is it possible for the atom to emit a photon in the visible part of the electromagnetic spectrum when the electron drops directly or cascades down to the ground state? ANSWER. Yes. The electron can drop from level n = 3 to level n = 2 and, in so doing, emit Hα, which is a Balmer series (visible) photon. 8.
Energy level diagrams are the representation of placements or arrangements of orbitals (also known as subshells) according to their increasing energy levels. Image will be uploaded soon. Above is the blank energy level diagram which can be used to represent the electrons for any atom under study.
Match the type of system or situation to the appropriate energy level diagram.eerotational states of a diatomic molecule such as O2a.aavibrational states of ...
Problem: Match the type of system or situation to the appropriate energy level diagram. nuclear (such as the nucleus of a carbon atom) ...
UML diagram types •Activity diagrams, which show the activities involved in a process or in data processing . •Use case diagrams, which show the interactions between a system and its environment. •Sequence diagrams, which show interactions between actors and the system and between system components.
Figure 3: An MO energy level diagram for H 2. The up and down arrows represent electrons that are spin up or spin down. Sigma bonding orbitals and antibonding orbitals can also be formed between p orbitals (figure 4). Notice that the orbitals have to be in phase in order to form bonding orbitals.
That energy must be exactly the same as the energy gap between the 3-level and the 2-level in the hydrogen atom. The last equation can therefore be re-written as a measure of the energy gap between two electron levels. The greatest possible fall in energy will therefore produce the highest frequency line in the spectrum.




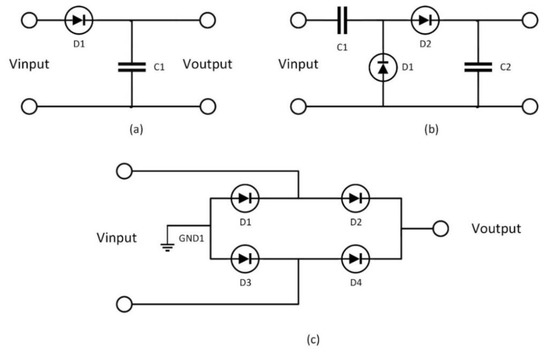

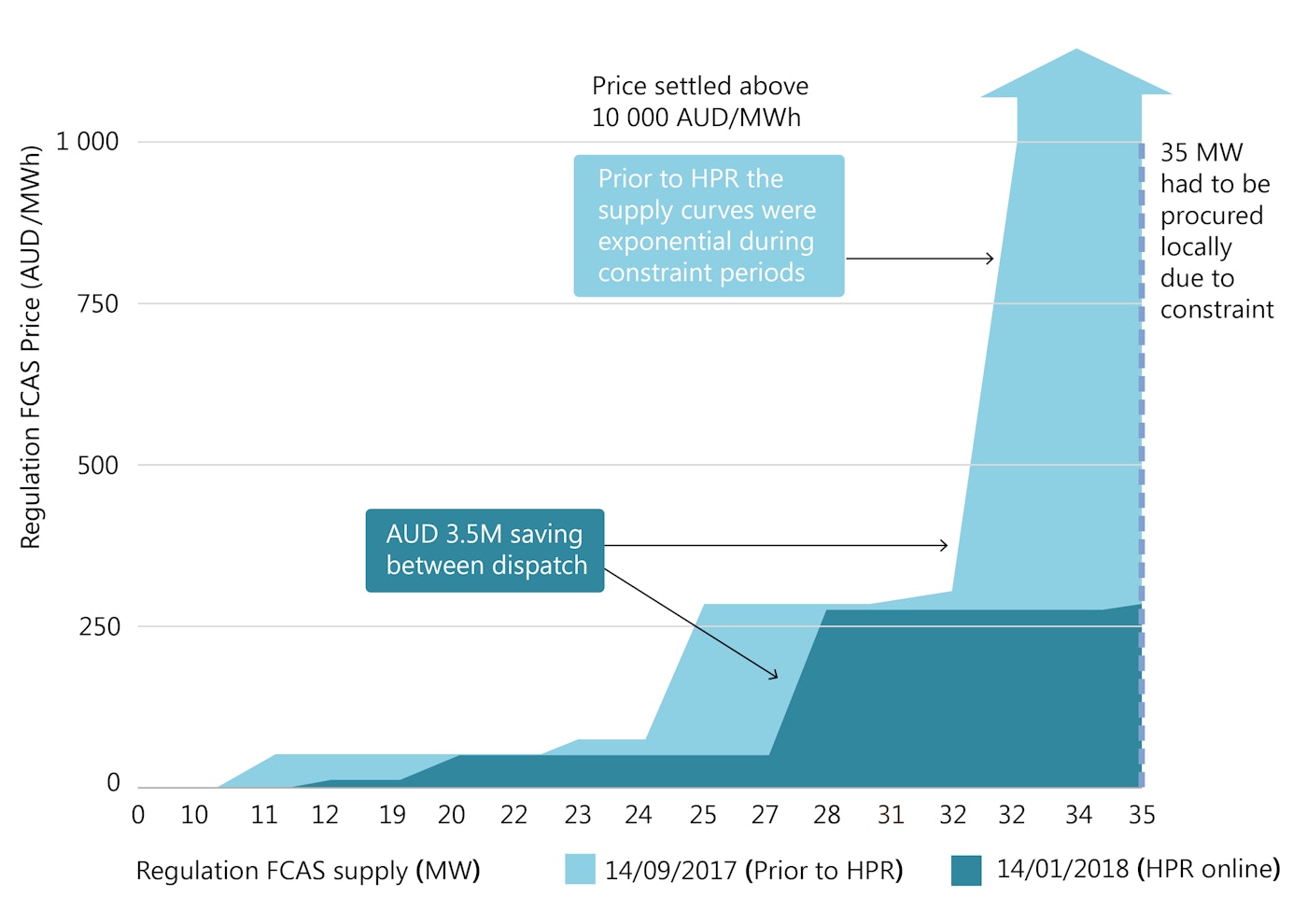
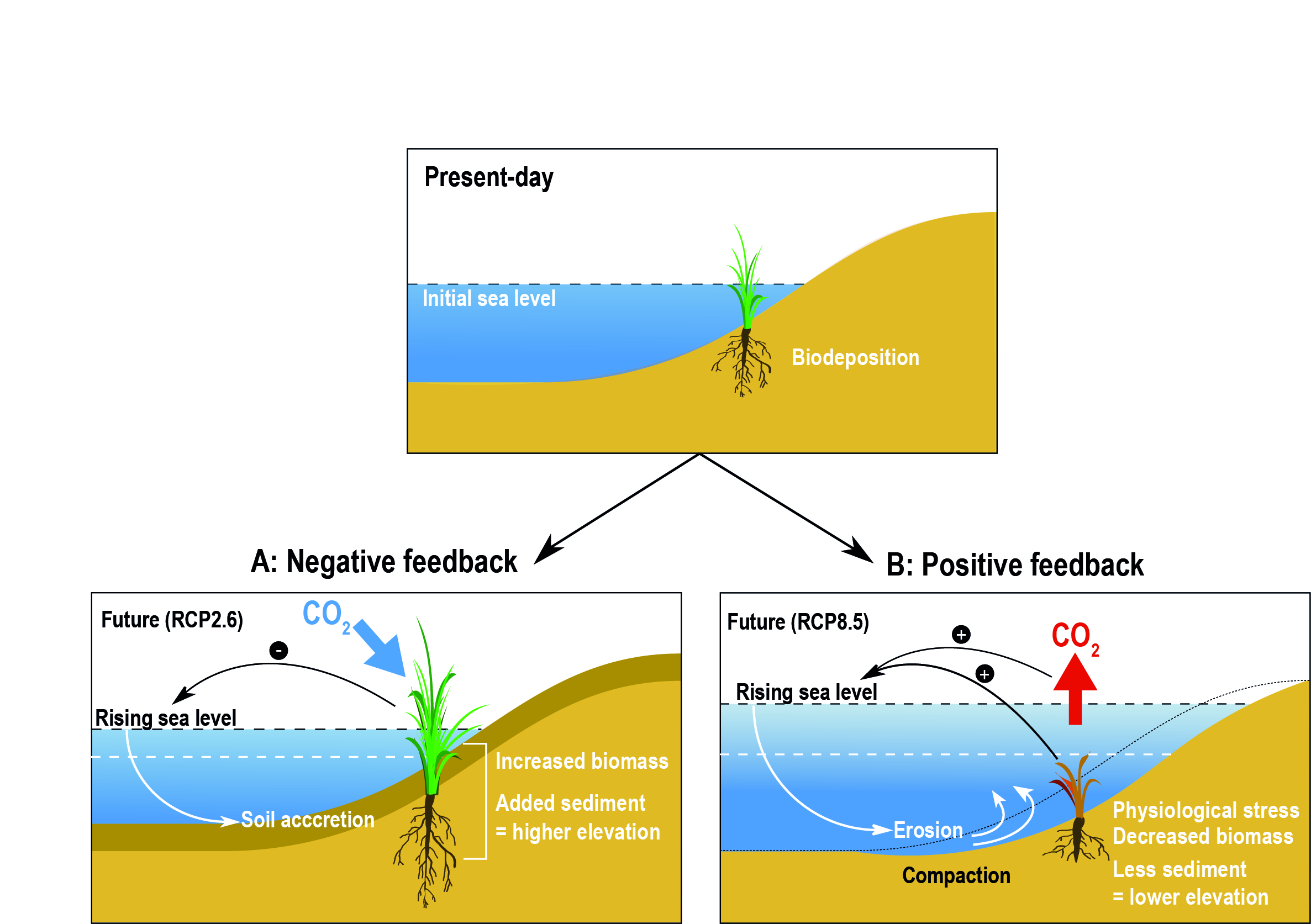




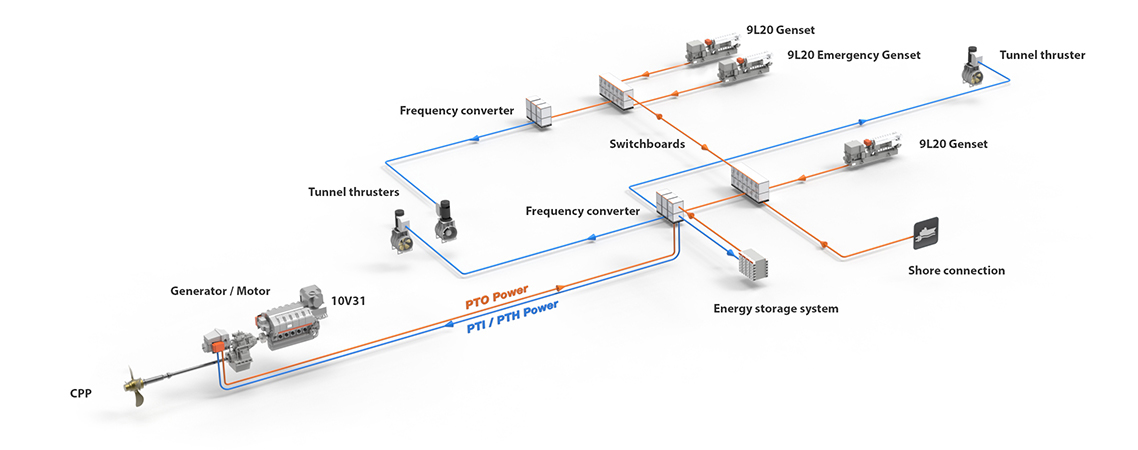

















0 Response to "41 match the type of system or situation to the appropriate energy level diagram."
Post a Comment