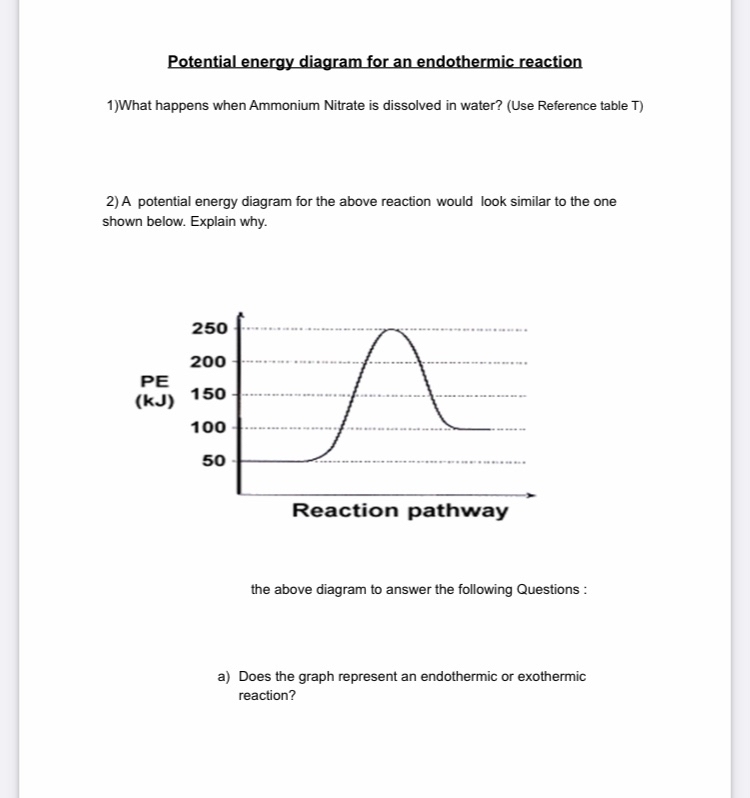
40 potential energy diagram endothermic
Energy Level Diagram of an Endothermic Reaction. The simple energy level diagram of endothermic and exothermic reactions are illustrated below. The activation energy is the energy that must be provided to the reactants so that they can overcome the energy barrier and react. For exothermic reactions, the potential energy of the product is ... ENERGY DIAGRAM. • A = POTENTIAL ENERGY OF REACTANTS. • B = ACTIVATION ENERGY. • ENERGY REQUIRED TO START THE REACTION. • C = ACTIVATION ENERGY (REVERSE.11 pages
Increase in kinetic energy - energy absorbed - endothermic Decrease in kinetic energy - energy released – exothermic Energy level diagrams Chemical Potential Energy The chemical potential energy stored in the bonds gives us a measure of a substances energy level. The higher the energy, the more chemical energy is stored in its bonds.

Potential energy diagram endothermic
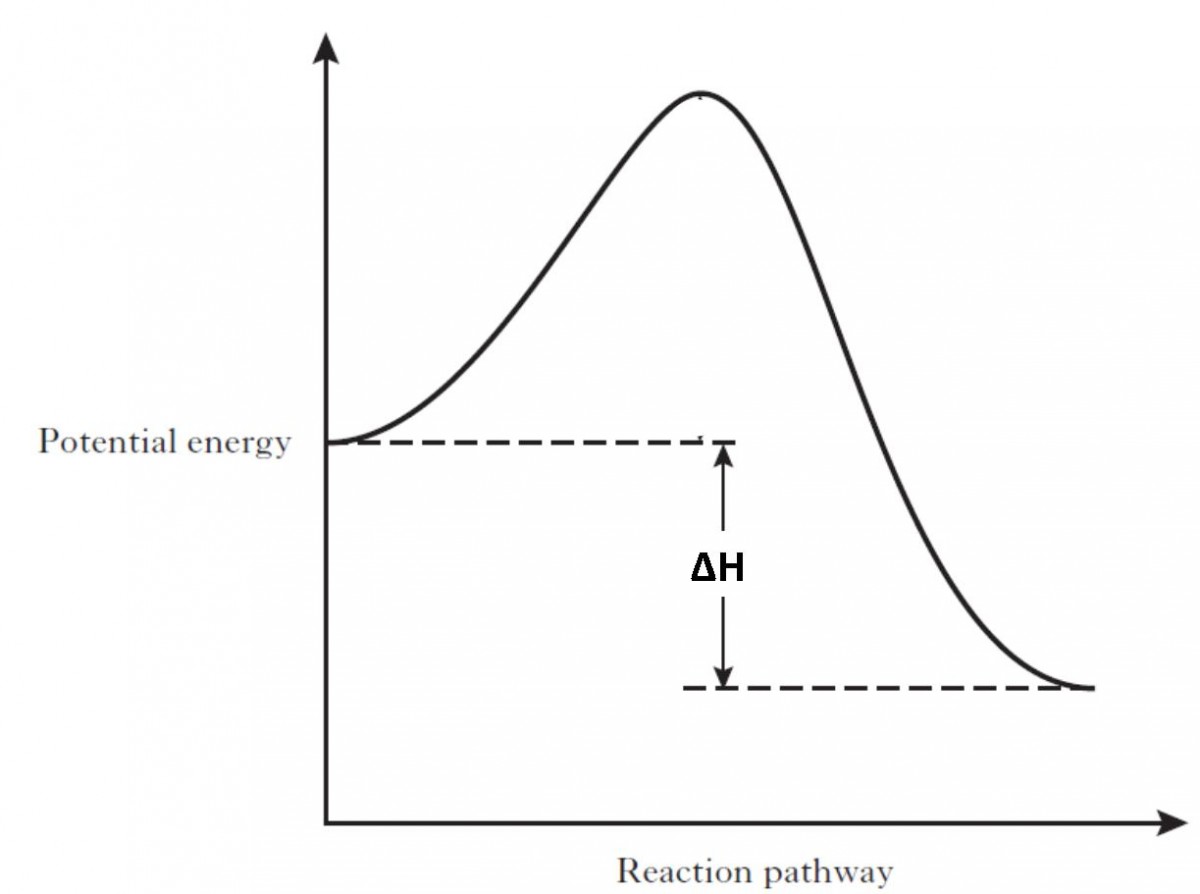
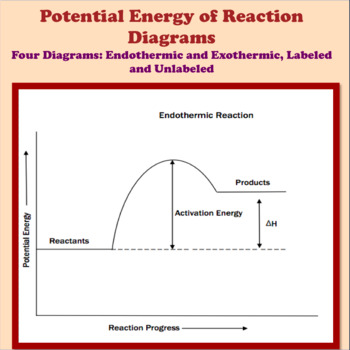
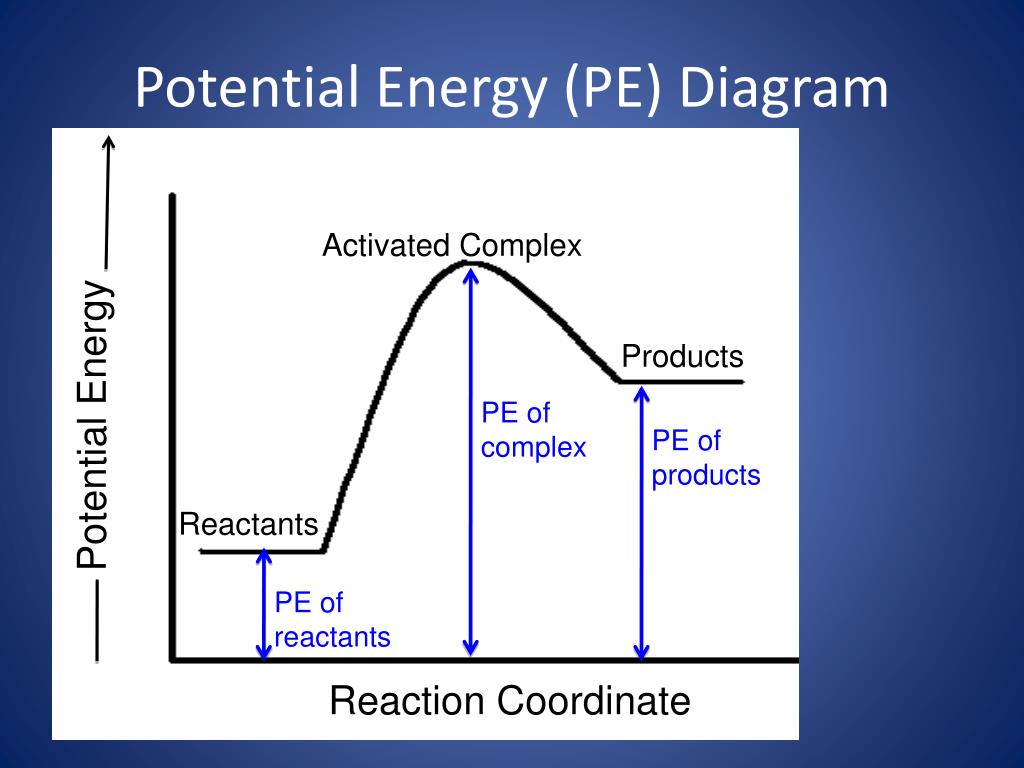
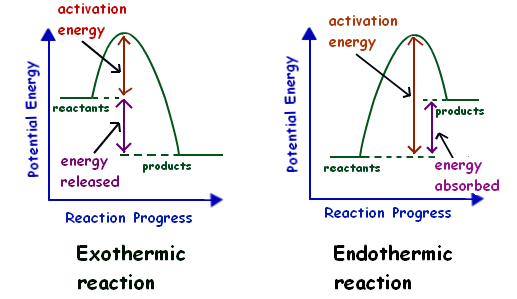
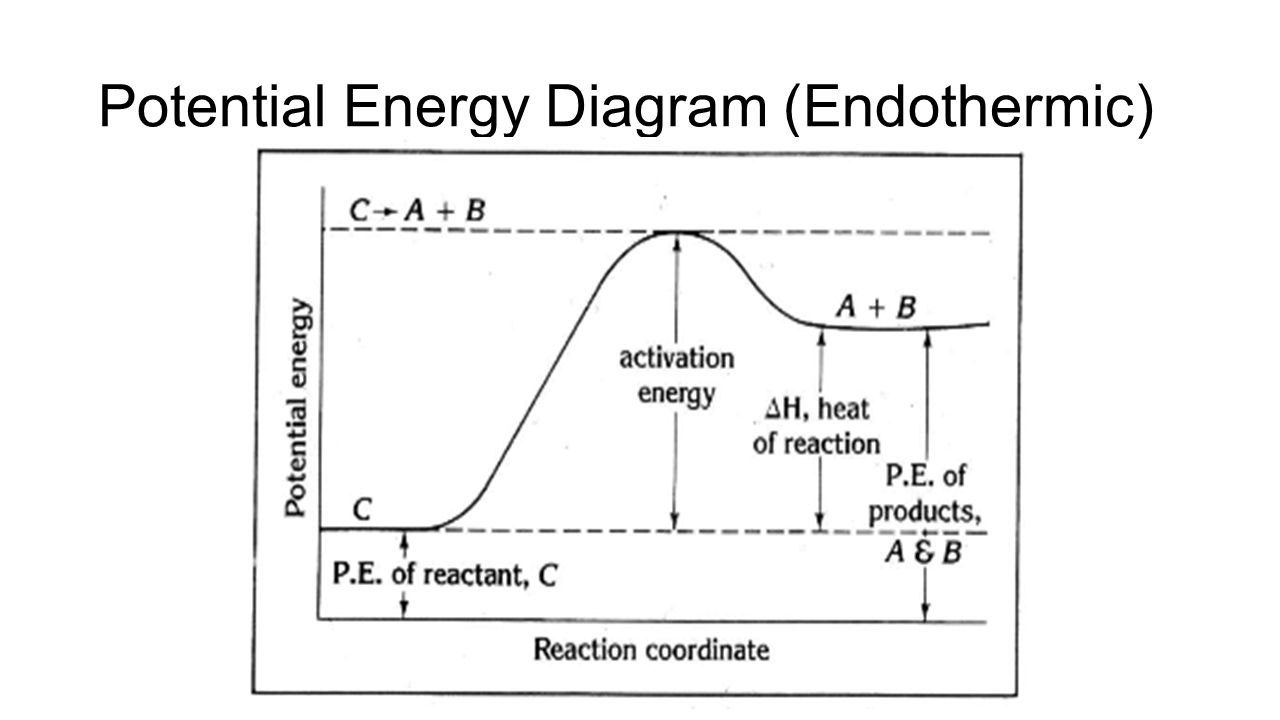
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. Potential energy diagrams. Chemical reactions involve a change in energy, usually a loss or gain of heat energy. The heat stored by a substance is called its enthalpy (H). \ (\Delta H\) is the ...
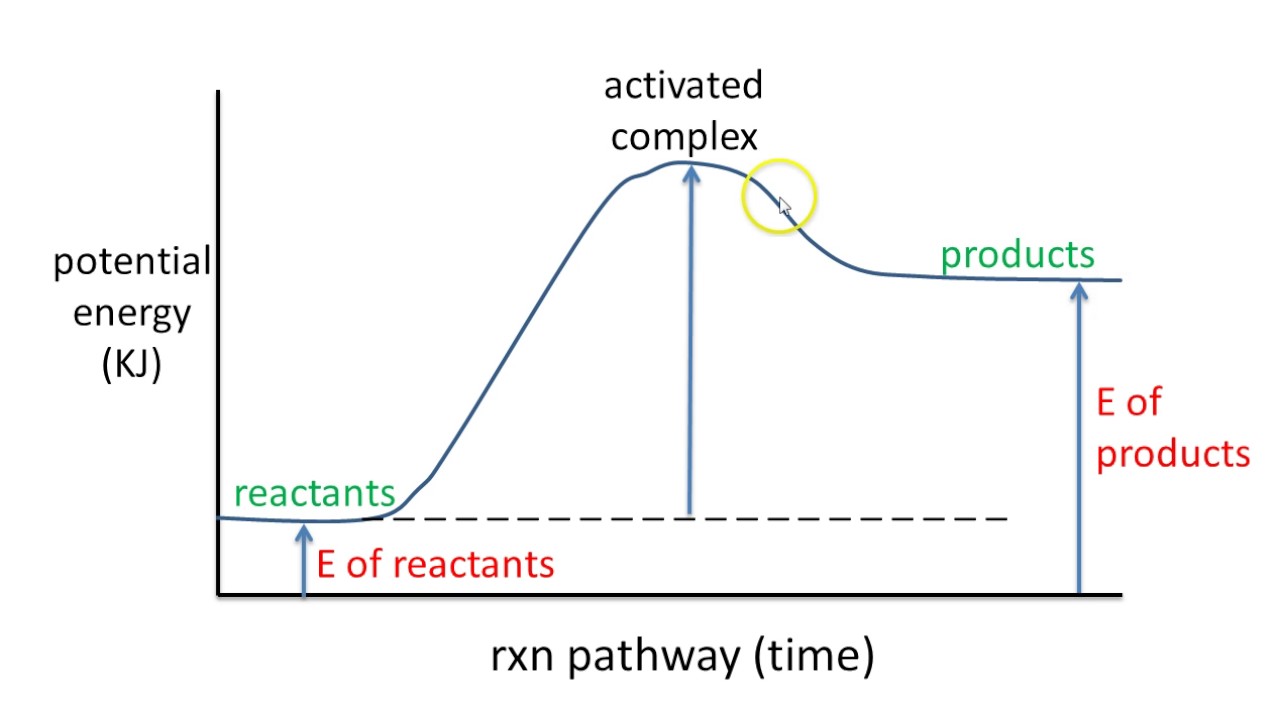
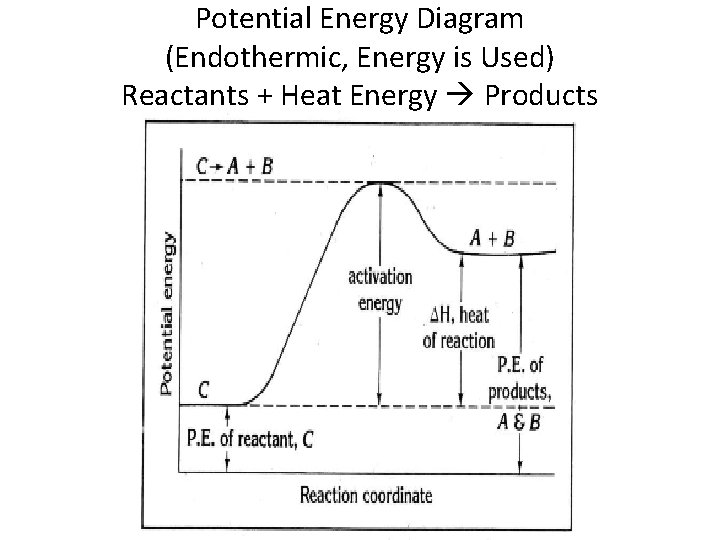
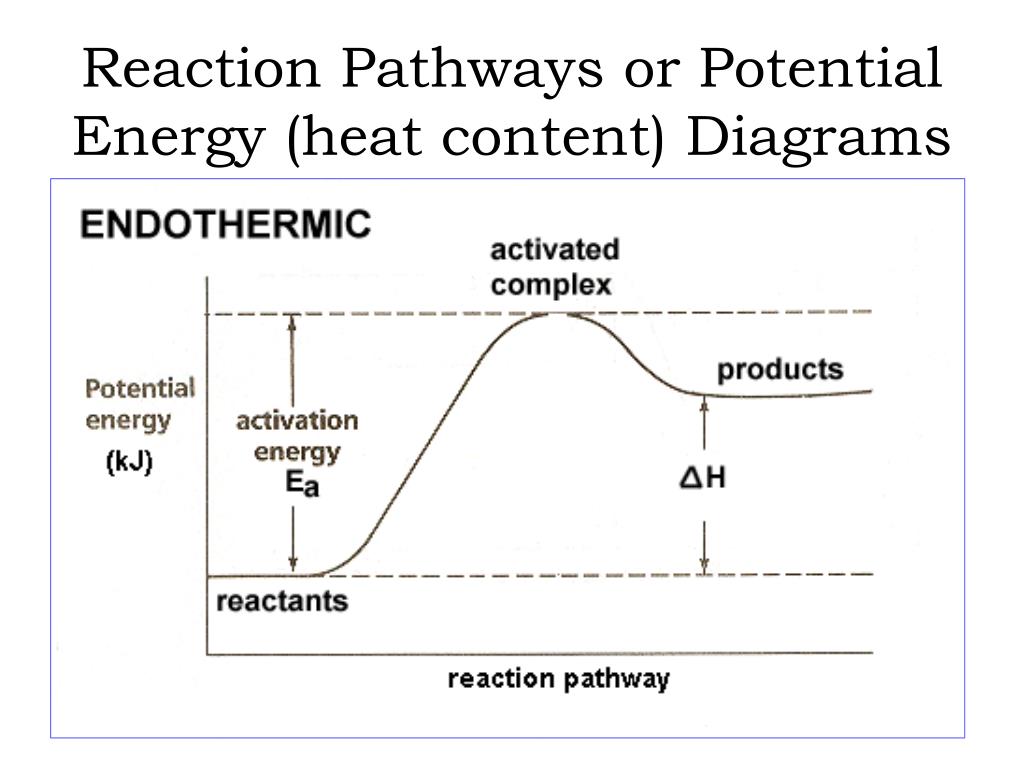
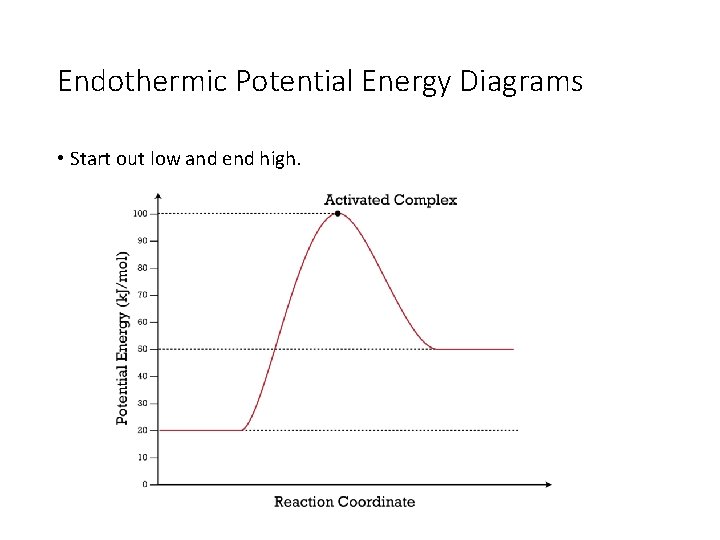
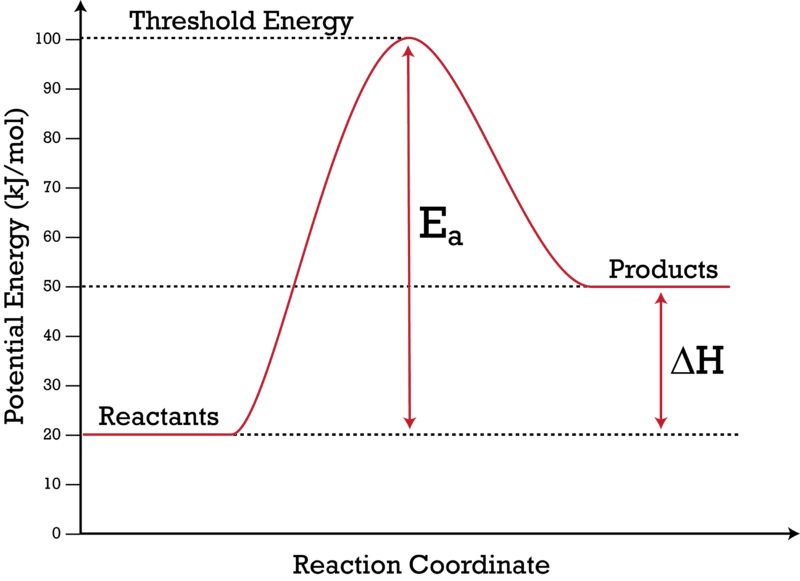
Potential energy diagram endothermic. In a potential energy diagram for an endothermic reaction, what best describes the difference in energy between reactants and products? a) Activation Energy b) Energy released c) Energy absorbed d) activated complex e) all of the above Energy is released in an exothermic reactions because: a) the potential energy of products is greater than ... Endothermic Reaction Energy Level Diagram: Endothermic reactions are depicted in a basic energy level diagram below. The activation energy is the amount of energy that must be delivered to the reactants for them to break through the energy barrier and react. In an endothermic reaction, the result has higher potential energy than the reactants. An endothermic reaction must have a positive change in enthalpy. That is, Delta"H">0. This means that the system absorbs heat. To represent the positive change in enthalpy, the potential energy of the reactants must be LOWER than that of the products. Notice how the end of the "hump" is higher than the beginning, showing how the system gained energy during the course of the reaction. Since the potential energy of the products is higher than the potential energy of the reactants, the reaction is endothermic and the enthalpy (E(C)-E(A)) has a positive sign.The activation energy (E(B)-E(A)) is positive as well.. We have a diagram showing the potential energy changes for a reaction pathway.. Point A represents the potential energy of the reactants.
3. Using the potential energy diagrams for an endothermic and exothermic reaction shown, choose the letter that best fits each statement. Reaction I Reaction 2 a) E P of the reactants C R b) E P of the products E Z c) ∆H of the reaction B S d) activation energy of the 52 Sketch the potential energy diagram for an endothermic chemical reaction that shows the activation energy and the potential energy of the reactants and the potential energy of the products. Answer--> 1/04. 16 Which statement best explains the role of a catalyst in a chemical reaction? Endothermic reactions take in energy and the temperature of the surroundings decreases. Energy is being put in to break bonds in the reactants. In this diagram the activation energy is signified by the hump in the reaction pathway and is labeled. Energy reactants products exothermic reactions the reactants have more potential energy than the ... 9 Jul 2019 — Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the ...
Transcribed image text: The reaction energy diagrams for an endothermic and an exothermic reaction are shown below. Observe the graphs, and classify the following properties of exothermic and endothermic reactions. Energy of reactants Activation energy Potential energy Potential energy Change in Energy of products Change in potential energy Activation ghergy Energy of reactants potential ... This chemistry video tutorial focuses on potential energy diagrams for endothermic and exothermic reactions. It also shows the effect of a catalyst on the f... is positive for an endothermic reaction and negative for an exothermic reaction. This can be seen in the potential energy diagrams. The total potential energy ... Representing a Reaction with a Potential Energy Diagram (Student textbook page 371) 11. Complete the following potential energy diagram by adding the following labels: an appropriate label for the x-axis and y-axis, E a(fwd), E a(rev), ΔH r. a. Is the forward reaction endothermic or exothermic? b.
23 Feb 2012 — Potential energy diagrams illustrate the potential energy of the reactants and products for a chemical reaction and how this energy changes ...
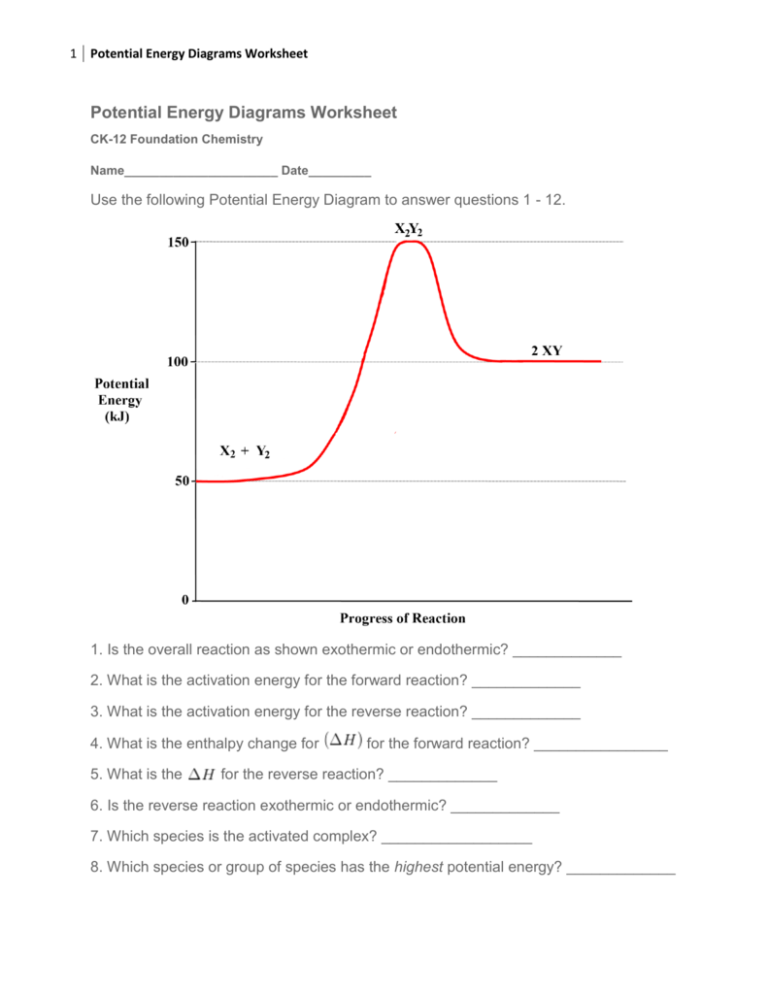
The reverse reaction is ____exothermic_____ (endothermic or exothermic). Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what ...
An energy level diagram. shows whether a reaction is exothermic. or endothermic. It shows the energy in the reactants and products , and the difference in energy between them. Exothermic reaction
Representing endothermic and exothermic processes using energy diagrams. AP.Chem: ENE‑2.B (LO) , ENE‑2.B.1 (EK) Transcript. A physical or chemical process can be represented using an energy diagram, which shows how the potential energy of the initial state relates to the potential energy of the final state.
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change \(\left( \Delta H \right)\) is positive for an endothermic reaction and negative for ...
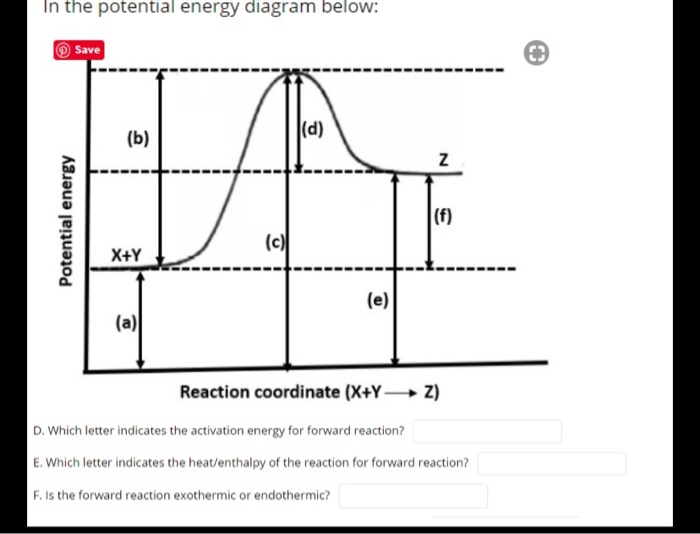
Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain whv.] According to the diagram, is the potential eneoy of the products greater than, less than, or equal to the potential energy of the reactants? Draw an alTOW on the diagram above to represent the activation energy for the forward reaction. Label the
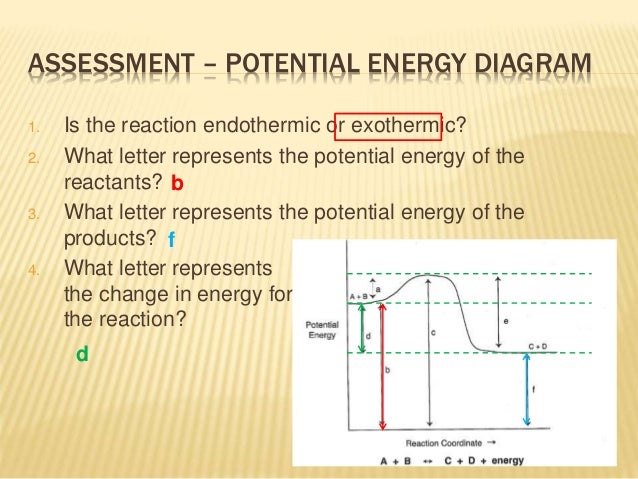
ASSESSMENT - POTENTIAL ENERGY DIAGRAM 1. Is the reaction endothermic or exothermic? 2. What letter represents the potential energy of the reactants? 3. What letter represents the potential energy of the products? 4. What letter represents the change in energy for the reaction? b f d 11. ASSESSMENT - POTENTIAL ENERGY DIAGRAM 5.
Chemistry*12* Potential*Energy*Diagrams*Worksheet* Name:* Date:* Block:*! USE!THE!POTENTIAL*ENERGY*DIAGRAM!TO!ANSWER!THEQUESTIONS!BELOW:! 1.! Is!the!overall!reaction ...
Schematic potential energy diagram showing the effect of a catalyst in an endothermic chemical reaction. Chemistry is the scientific study of the properties and behavior of matter. The loss of this electric potential energy in the external circuit results in a gain in light energy thermal energy and other forms of non-electrical energy.
Energy level diagrams are used to shows the energy content of chemicals before and after a reaction. They show: (a) the total energy content of the reactants compared to the total energy content of the products. Figure shows the energy level diagram of an exothermic reaction. Figure shows the energy level diagram of an endothermic reaction.
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction ...
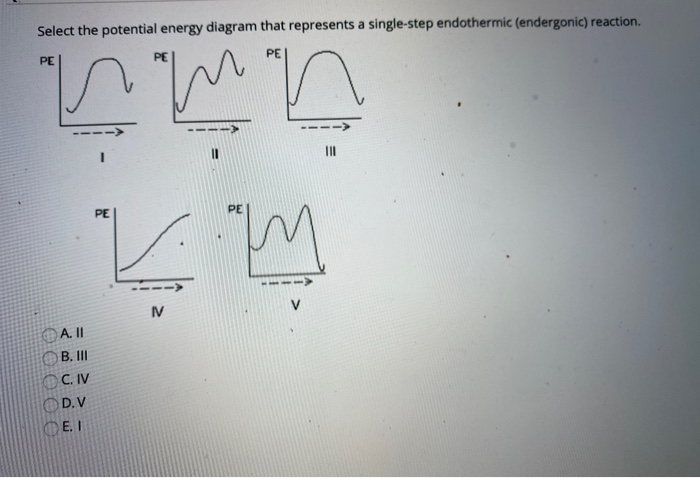
7. Be able to recognize and read potential energy diagrams. Reaction Coordinate Reaction Coordinate Exothermic Endothermic "downhill" "uphill" 8. ∆H is (+) for endothermic reactions and is (-) for exothermic reactions. 9. The rates of the forward and reverse reactions are equal at equilibrium. 10.
This chemistry video tutorial provides a basic introduction into endothermic and exothermic reactions as well as the corresponding potential energy diagrams....
Enthalpy, or heat energy, is represented by ΔH (Δ is the delta sign, which means change).If there is a negative change in energy, or -ΔH, an exothermic reaction is taking place and energy is released🔥 from the system to the surroundings. If there is a positive change in energy, or +ΔH, an endothermic reaction is taking place and energy is absorbed into the system from the surroundings.
Potential energy diagrams. Chemical reactions involve a change in energy, usually a loss or gain of heat energy. The heat stored by a substance is called its enthalpy (H). \ (\Delta H\) is the ...
1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process.
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change (ΔH) is positive for an endothermic reaction and negative for an exothermic reaction.

















0 Response to "40 potential energy diagram endothermic"
Post a Comment