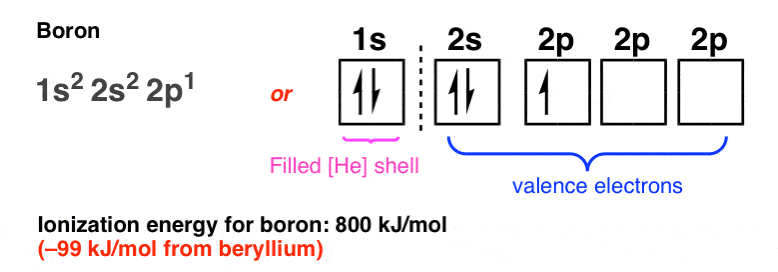
45 orbital filling diagram for boron
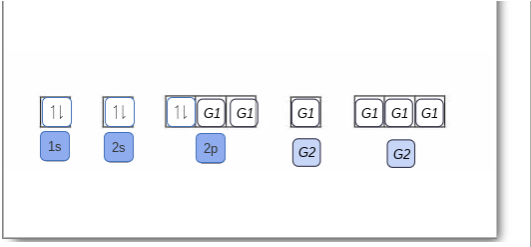
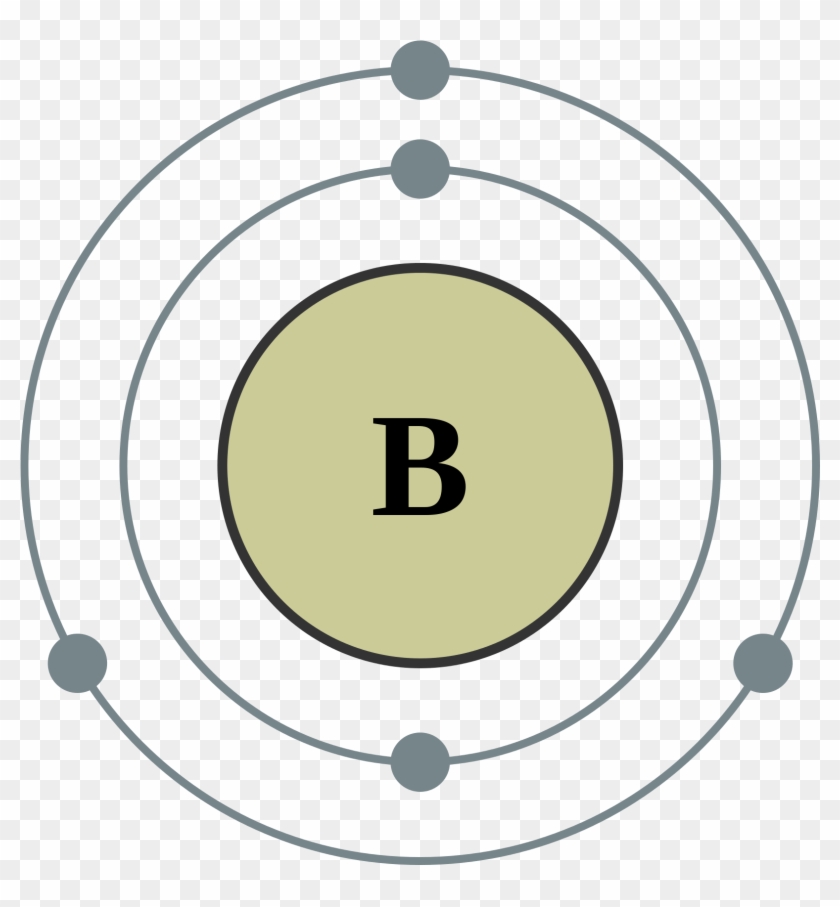
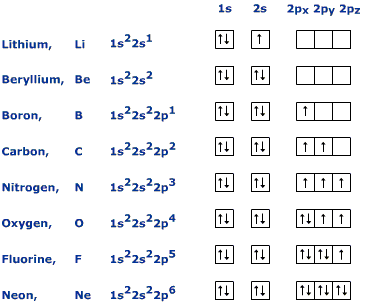
The orbital filling diagram of boron. I skipped past beryllium because I was getting bored. The electron configuration of boron is 1s²2s²2p¹, which means that there are two electrons in the 1s orbital, two electrons in the 2s orbital, and one electron in the 2p orbitals. This gives us an orbital filling diagram of: Boron is the fifth element with a total of 5 electrons. The electron configuration of boron is 1s²2s²2p¹ which means that there are two electrons in the 1s orbital two electrons in the 2s orbital and one electron in the 2p orbitals. This gives us an orbital filling diagram of. The remaining electron will go in the 2p orbital.
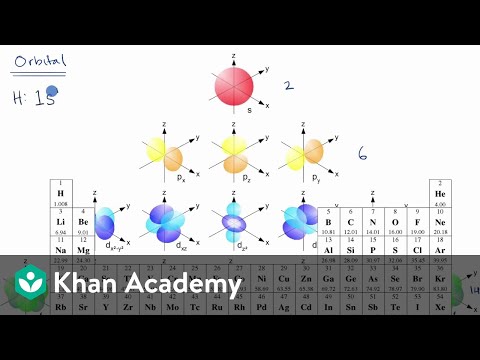
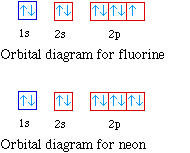
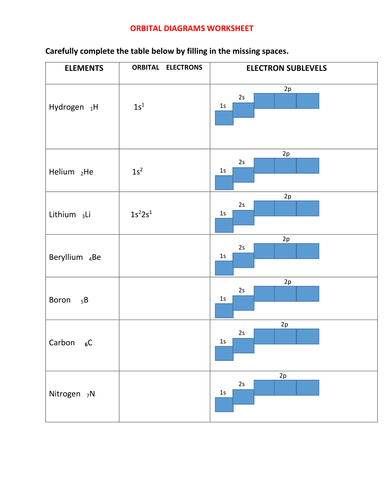
Orbital Diagram of All Elements Diagrams; 1: Orbital diagram of Hydrogen (H) 2: Orbital diagram of Helium (He) 3: Orbital diagram of Lithium (Li) 4: Orbital diagram of Beryllium (Be) 5: Orbital diagram of Boron (B) 6: Orbital diagram of Carbon (C) 7: Orbital diagram of Nitrogen (N) 8: Orbital diagram of Oxygen (O) 9: Orbital diagram of Fluorine ...

Orbital filling diagram for boron
1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium Orbital filling diagram of boron? Boron has:- 1s2 2s2 2p1. What is hydrogen's orbital notation? Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a ... Boron has:- 1s2 2s2 2p1. Why are the outermost electrons the only ones included in the orbital filling diagram and the electron dot diagram?
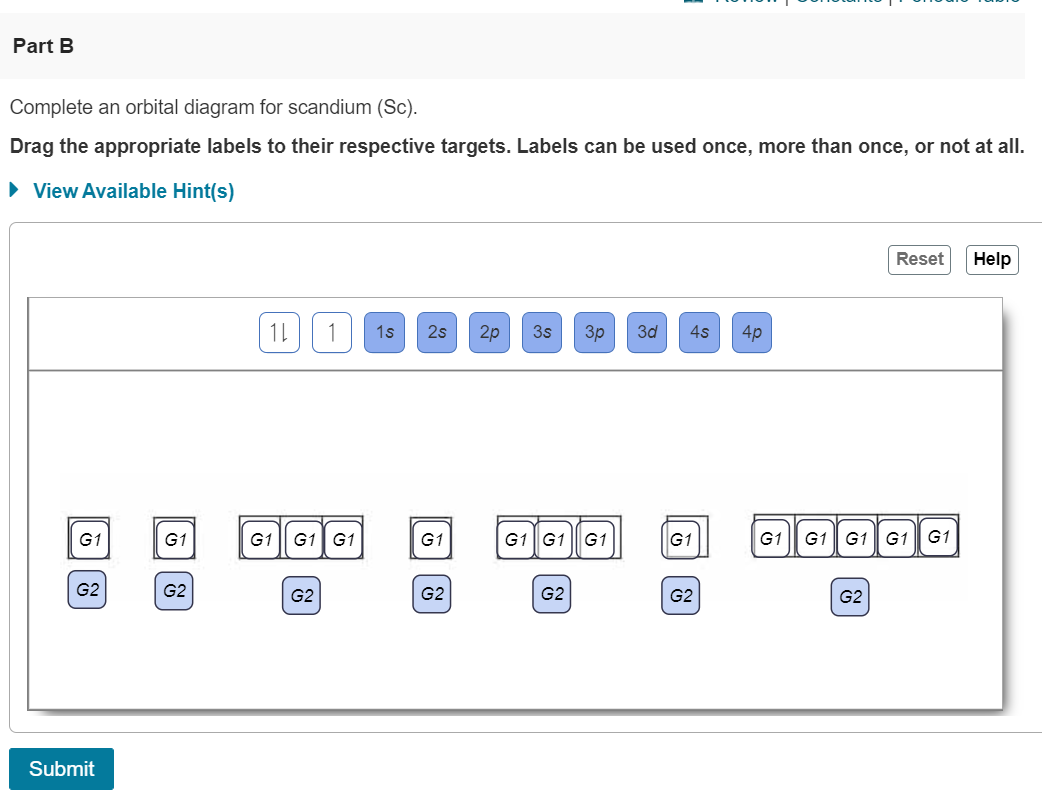
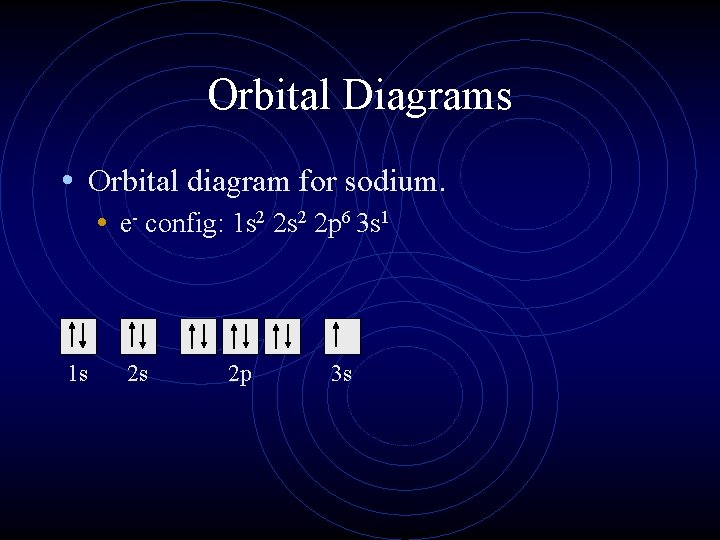
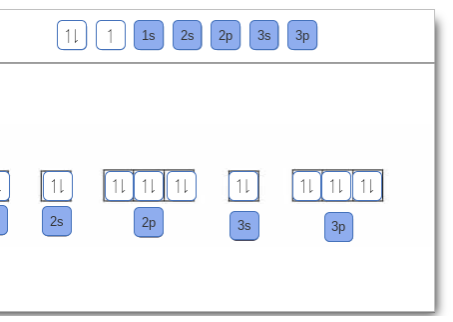
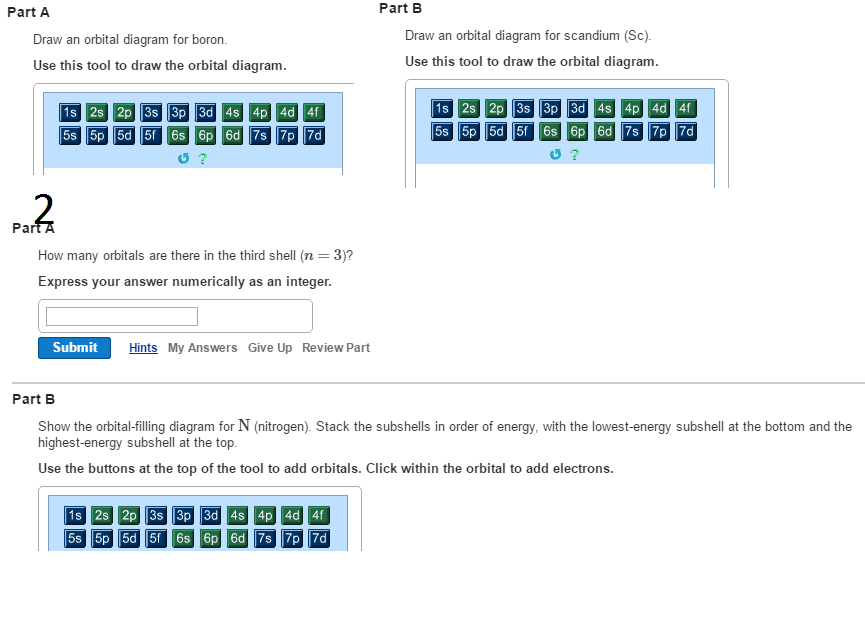
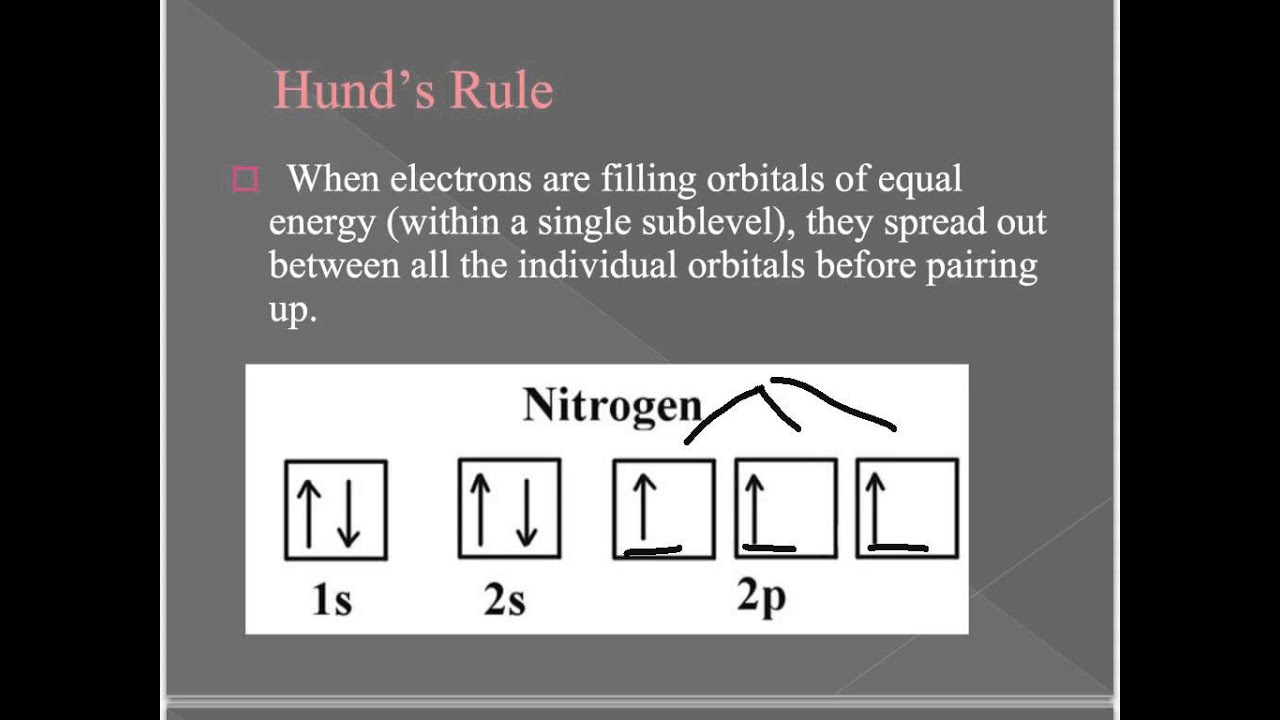
Orbital filling diagram for boron. Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). From the orbital diagram, we can write the electron configuration in an abbreviated When we reach boron, with Z = 5 and five electrons, we must place the fifth . After filling the first five rows, we still have 80 − 54 = 26 more.Orbital Filling Diagrams. Orbital filling diagram for boron. The electron configuration of boron is 1s²2s²2p¹ which means that there are two electrons in the 1s orbital two electrons in the 2s orbital and one electron in the 2p orbitals. Boron has 1s2 2s2 2p1. Boron is the fifth element with a total of 5 electrons. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of energy, with the lowest-energy subshell at the bottom and the highest-energy subshell at the top. Use the buttons at the top of the tool to add %(8). Question: Orbital Diagrams Draw an orbital diagram for boron. Use this tool to draw the orbital diagram. Draw an orbital diagram for scandium (Sc). Use this tool to draw the orbital diagram. How many orbitals are there in the third shell (n = 3)? Express your answer numerically as an integer. Show the orbital-filling diagram for N (nitrogen). Stack the subshells in order of. Question: Draw an orbital diagram for boron.
Boron has:- 1s2 2s2 2p1. Why are the outermost electrons the only ones included in the orbital filling diagram and the electron dot diagram? Orbital filling diagram of boron? Boron has:- 1s2 2s2 2p1. What is hydrogen's orbital notation? Orbital notation shows the number of electronics in an orbit. The orbital notation of Hydrogen is a ... 1. Write orbital filling diagrams, electron configurations, and electron dot diagrams for the following elements. Table: Element Orbital Filling Diagram Electron Configuration Electron Dot Diagram a. Boron b. Silicon c. Sulfur d. Calcium e. Iodine f. Rubidium g. Chromium h. Gallium

Electron Configurations And Orbital Diagrams Principles For Filling Orbitals Writing Electron Configurations Ppt Download

Review I Constants I Show The Orbital Filling Diagram For S Sulfur Order Subshells By Energy With Homeworklib















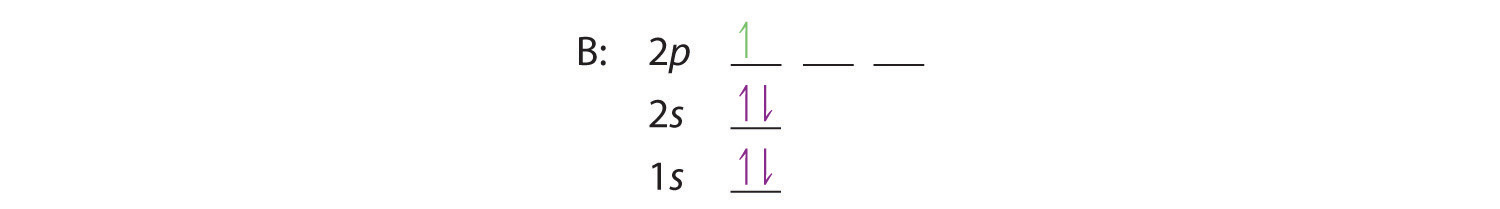







0 Response to "45 orbital filling diagram for boron"
Post a Comment