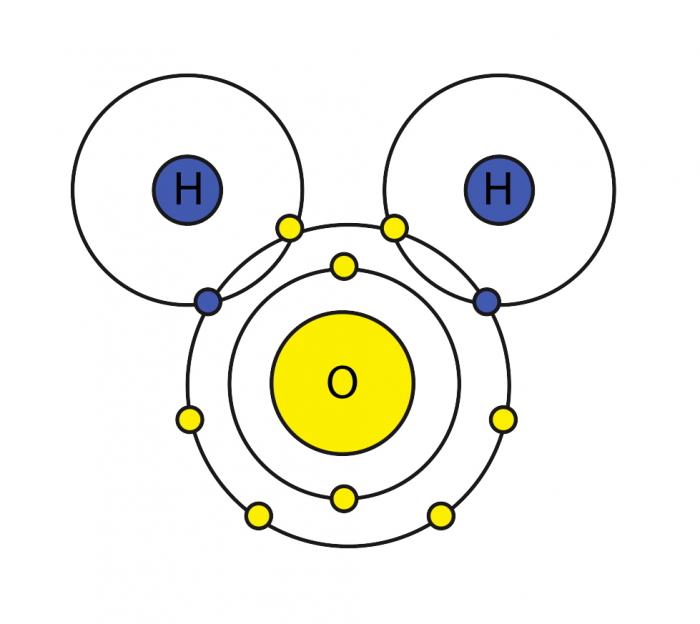
44 make an electron distribution diagram of water
Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) - 3 - •• 0 : 19. Another bond type is the 1)Draw an electron-dot diagram for each of the following substances: a CaO(an ionic compound) b HBr c N2 Base your answers to questions 2 and 3 on the information below and on your knowledge of chemistry. The formulas and the boiling points at standard pressure for ethane, methane, methanol, and water are shown in the table below.
The molecule of water. A molecule is an aggregation of atomic nuclei and electrons that is sufficiently stable to possess observable properties — and there are few molecules that are more stable and difficult to decompose than H 2 O. In water, each hydrogen nucleus is bound to the central oxygen atom by a pair of electrons that are shared between them; chemists call this shared electron pair ...
Make an electron distribution diagram of water
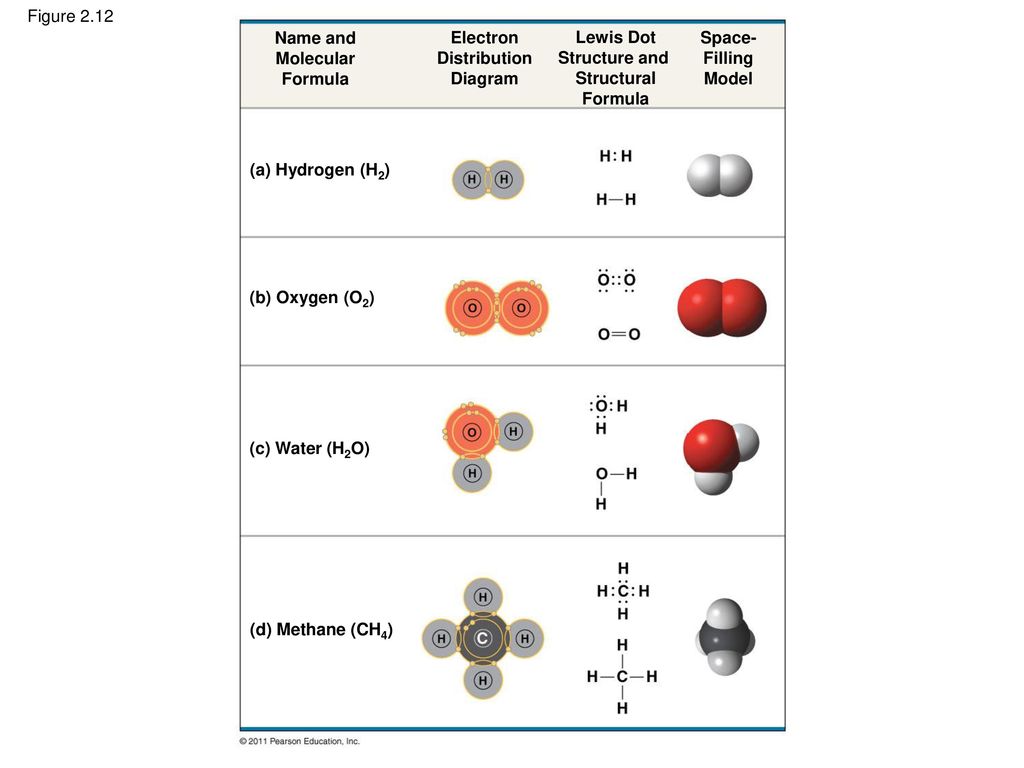
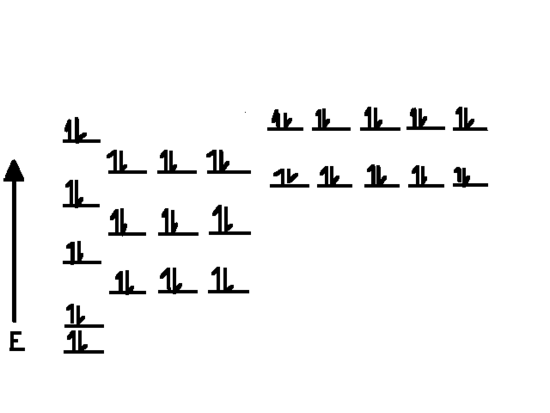
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n Structure of water molecule is made up of one molecule of oxygen and two molecules of hydrogen bonded covalently. Water (H 2 O) essentially considered one of the most important substances found on the earth. It covers over 70% of the earth's surface and makes up as much as 95% of the living organisms. It is virtually unique among liquids ... Electron-distribution diagram Atomic mass 2 He 4.00 First shell Second shell Third shell Sodium 11 Na Magnesium 12 Mg Aluminum 13 Al Silicon 14 Si ... Water (H 2 O) Name and Molecular Formula Electron-distribution Diagram Lewis Dot Structure and Structural Formula Space-filling Model. Fig. 2-12d (d) Methane (CH 4)
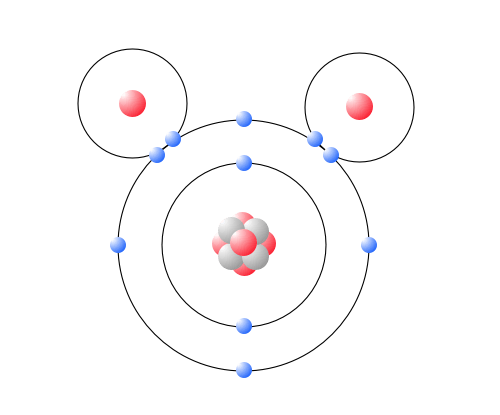
Make an electron distribution diagram of water. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a . polar . molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) The Configuration of the Water Molecule. A molecule of water is composed of two atoms of hydrogen and one atom of oxygen. The one and only electron ring around the nucleus of each hydrogen atom has only one electron. The negative charge of the electron is balanced by the positive charge of one proton in the hydrogen nucleus. Electron Configuration Diagrams | Properties of Matter | Chemistry | FuseSchoolLearn the basics about Drawing electron configuration diagrams. Find out more ... For each electron shell atom diagram, the element symbol is listed in the nucleus. The electron shells are shown, moving outward from the nucleus. The final ring or shell of electrons contains the typical number of valence electrons for an atom of that element. The element atomic number and name are listed in the upper left.
Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) 1. Another bond type is the ionic bond. Explain what is happening in Figure 2.10. For example, the bond between the oxygen and hydrogen atoms of a water molecule is a polar covalent bond. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Water is an oxygen hydride consisting of an oxygen atom that is covalently bonded to two hydrogen atoms. It has a role as an amphiprotic solvent, a member of greenhouse gas, a human metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite and a mouse metabolite. Make an electron distribution diagram of water. Due to the uneven distribution of the electrons you create slightly aka delta positive and slightly negative regions in the molecule. Why is water considered a polar molecule. Water supply service diagram mo diagram of difluorine aufbau diagram electron distribution diagram lewis dot structure and ...
The figure below represents an electron distribution diagram where the concentric circles represent electron shells and each small circle represents an empty spot for an electron. Fill out the electron distribution diagram for the element carbon by coloring the electrons that are present; then answer the questions. Figure 2.9 Electron distribution diagrams for the first 18 elements in the periodic table. Figure 2.10 Electron orbitals. Figure 2.11 Formation of a covalent bond. Figure 2.12 Covalent bonding in four molecules. Figure 2.13 Polar covalent bonds in a water molecule. Figure 2.14 Electron transfer and ionic bonding. Answer (1 of 3): Depends on the theory chosen, Valence Bond Theory considers sp³ hybridization of valence shell of oxygen, where two orbitals have electron pairs shared with hydrogen atom and two lone orbitals with lone pairs. Molecular Orbital Theory, in other hand, depicts each pair of electro... make an electron distribution diagram of water. Subject: Biology Price: Bought 3. Share With. make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative
In this video we discuss the structure of water. We cover how and why is water a solvent to other substances, and how the ability of water to act as a solve...
18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
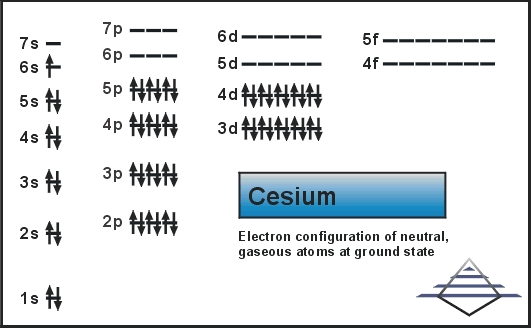
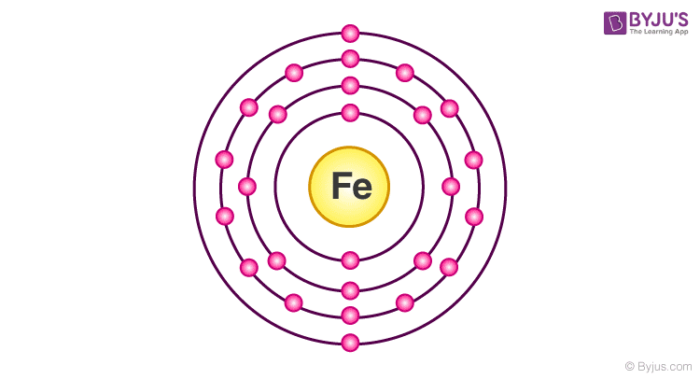
energy needed to remove an electron from an atom in the gas phase -Representation: Na (g) Na+ (g) + e -IE for this 1st ionization = 495.8 kJ/mol •In general, ionization energy increases as Z eff increases -Exceptions occur due to the stability of specific electron configurations
18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
The spatial distribution of electrons occupying each of these orbitals is shown in the diagram below. The valence shell electron configuration of carbon is 2 s 2, 2p x 1, 2p y 1 & 2p z 0. If this were the configuration used in covalent bonding, carbon would only be able to form two bonds.
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element's symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) 19. Another bond type is the ionic bond. Explain what is happening in the figure below ...
Shape of water molecule Lewis dot diagram O H 104.5o H space filling model. O-H bonds are polarized because of the difference in electronegativity between the O and H atoms. Hydrogen bonds This unequal electron distribution results in strong non-bonding interactions between water molecules - hydrogen bonds.
18. Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!) 19. Another bond type is the ionic bond. Explain what is happening in the figure below ...
3. Make an electron distribution diagram of carbon. ! Carbon has 4 valence electrons, can bond to 4 items, and typically forms covalent bonds with other elements. 4. Carbon chains form skeletons. List here the types of skeletons that can be formed. Carbon skeletons vary in length. The skeleton may have double bonds, which can vary in location.
Make an electron distribution diagram of water. Which element is most electronegative? Why is water considered a polar molecule? Label the regions that are more positive or more negative. (This is a very important concept. Spend some time with this one!)
Electron-distribution diagram Atomic mass 2 He 4.00 First shell Second shell Third shell Sodium 11 Na Magnesium 12 Mg Aluminum 13 Al Silicon 14 Si ... Water (H 2 O) Name and Molecular Formula Electron-distribution Diagram Lewis Dot Structure and Structural Formula Space-filling Model. Fig. 2-12d (d) Methane (CH 4)
Structure of water molecule is made up of one molecule of oxygen and two molecules of hydrogen bonded covalently. Water (H 2 O) essentially considered one of the most important substances found on the earth. It covers over 70% of the earth's surface and makes up as much as 95% of the living organisms. It is virtually unique among liquids ...
Bohr Diagrams 1) Check your work. 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1st shell. 4) The 2nd shell can hold up to 8 electrons. 5) The 3rd shell can hold 18, but the elements in the first few periods only use 8 electrons. 6p 6n




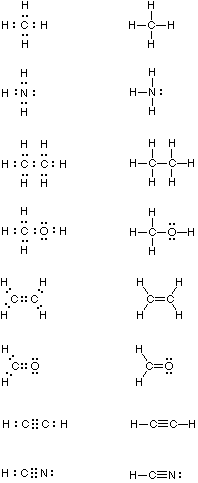




/atom--illustration-713786859-5bdb6f7d46e0fb002d6db6df.jpg)


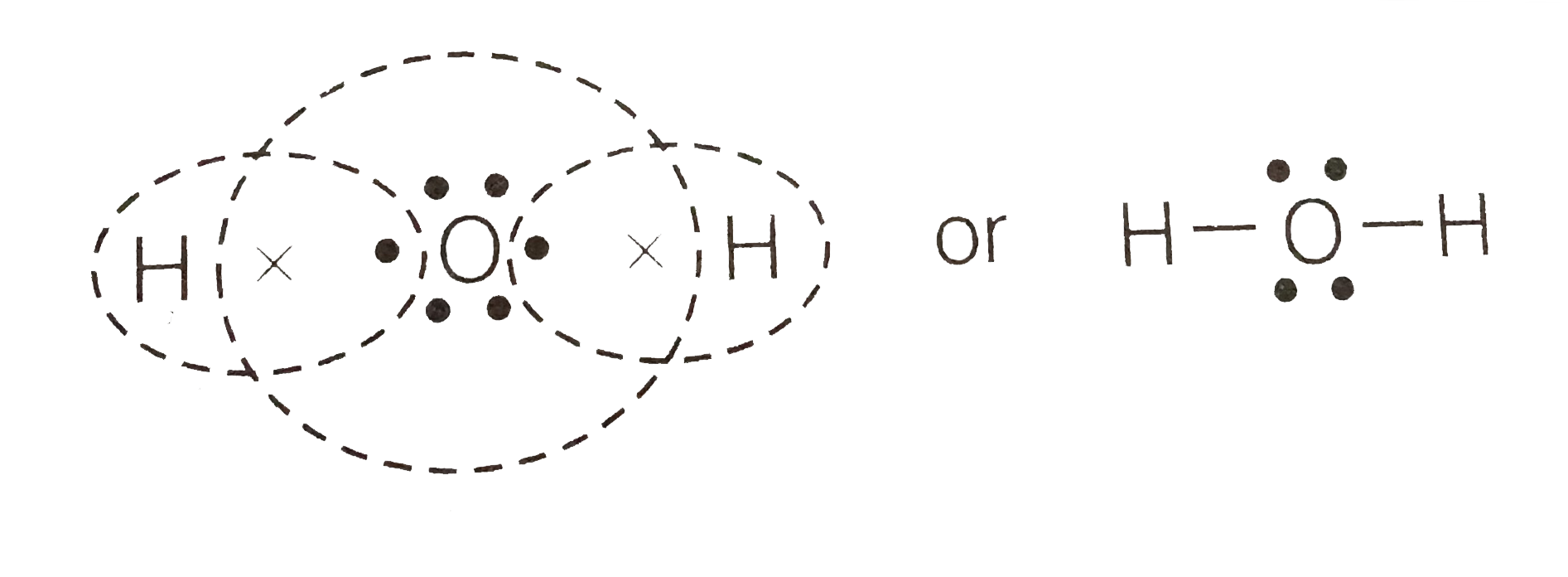











0 Response to "44 make an electron distribution diagram of water"
Post a Comment