44 given the potential energy diagram for a reaction
41 Given the potential energy, diagram for a reversible chemical reaction: Each interval on the axis labeled "Potential Energy (kJ/mol)'" represents 10. kilo joules per mole. What is the activation energy or the forward reaction? ( I ) 10. kJ/mol (2) 30. kJ/mol (3) 40. kJ/mol (4) 60. kJ/mol: 3: link: The energy to get up the hill. 4 boxes times ... The potential energy diagram for a reaction R → P is given below. ... Consider the potential energy diagram given below In accordance with Hammond's postulate, exothermic reactions tend to have _____. Hard. View solution > The threshold energy of the reaction is: Medium. View solution >
Potential Energy Progress of Reaction 26. Given the following mechanism, answer the questions below: Step 1: O 3 + NO ° NO 2 + O 2 (slow) Step 2: NO 2 + O ° NO + O 2 (fast) a) Give the equation for the overall reaction .

Given the potential energy diagram for a reaction
The reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) has ΔH°overall = -226 kJ/mol. A proposed reaction mechanism is shown. Choose the statement (s) that accurately describe the reaction energy diagram for the above reaction. -There will be three peaks. -The Ea of the first step will be larger than the second or third step. 11.Given the potential energy diagram for a reaction: Which intervals are affected by the addition of a catalyst? A)It is endothermic, and entropy decreases. B)It is endothermic, and entropy increases. C)It is exothermic, and entropy decreases. D)It is exothermic, and entropy increases. 12.Given the balanced equation representing a phase change: HELP ASAP I WILL GIVE BRAINLIST The potential energy diagram for a reaction starts at 380 kJ and ends at 100 k… Get the answers you need, now!
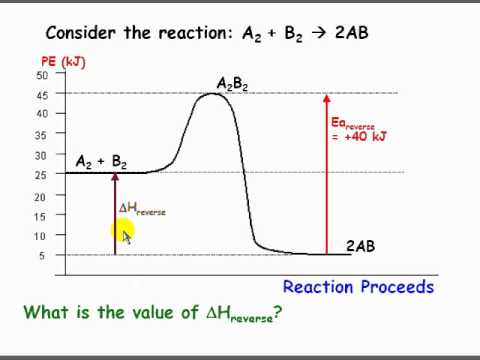
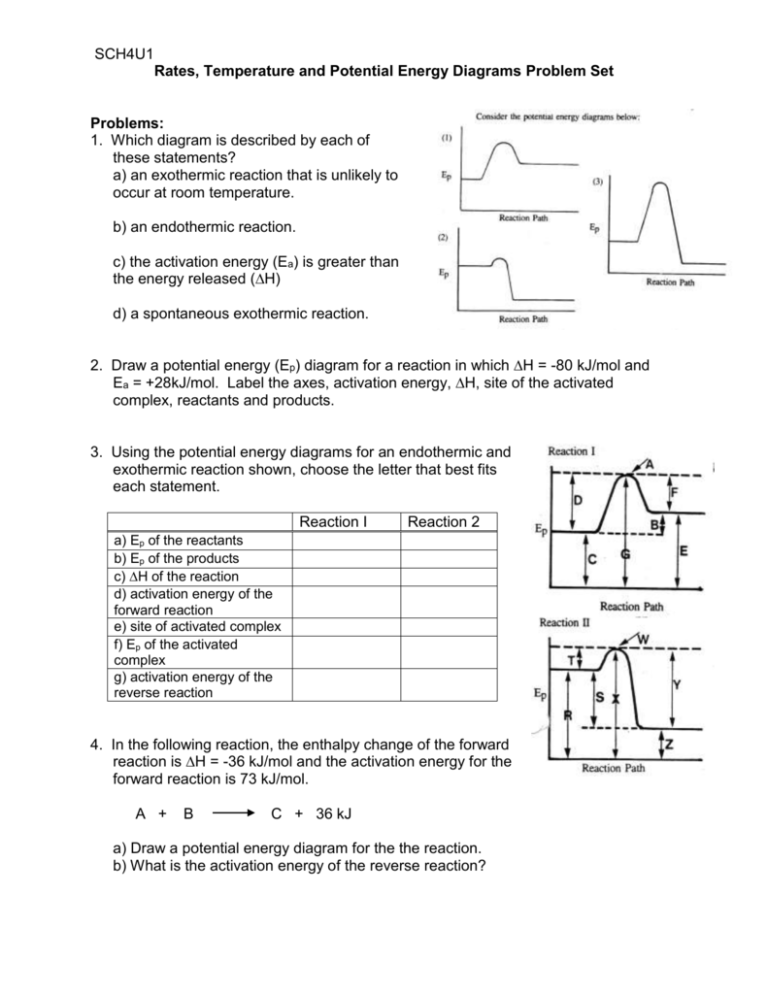
Given the potential energy diagram for a reaction. 4. Sketch a potential energy diagram for a general reaction A + B C + D Given that ΔH reverse = -10 kJ and E a forward = +40 kJ 5. Shown below is an energy diagram ... Given that a reaction absorbs energy and has an activation energy of 50 kJ/mol, which of the following statements are correct? (Hint: Draw the potential energy diagram.) (1) The reverse reaction has an activation energy equal to 50 kJ/mol. (2) The reverse reaction has an activation energy less than 50 kJ/mol. The potential energy diagram for a reaction `R rarr P` is given by Delta H^(@)` of the reaction compounds to the energy The potential energy diagram below represents a reaction. PE 40 Potential Energy Diagram pathway without with catalyst Course Ca culate the heat o reaction. What effect does a catalyst have in a ... potential energy that occur during the given reaction. Given the reaction: A + B --> C a) Does the diagram illustrate an exothermic or an ...
Reaction Rates and Potential Energy Diagrams 1. Chemical reactions occur when reactants collide. For what reasons may a collision fail to produce a chemical reaction? Not enough energy; improper angle. 2. If every collision between reactants leads to a reaction, what determines the rate at which Unit 1 - Reaction Kinetics d) Given that the uncatalyzed overall reaction is a slow exothermic reaction, draw a potential energy grcph which shows the possible shape of the curve for the uncatalyzed reaction. On the same graph, show a possible curve for the catalyzed reaction. Potential Energy u Progress of Reaction 3. 4. 5. Potential energy diagrams illustrate the potential energy of the reactants and products for a chemical reaction and how this energy changes during a reaction. Take a look at the potential energy diagram of the reaction shown below. The -axis represents the potential energy. The potential energy measures the energy stored within the bonds and ... The potential energy diagram and balanced equation shown below represent a reaction between solid carbon and hydrogen gas to produce 1 mole of C2H4(g) at 101.3 kPa and 298 K. Identify one change in the reaction conditions, other than adding a catalyst, that can increase the rate of this reaction.
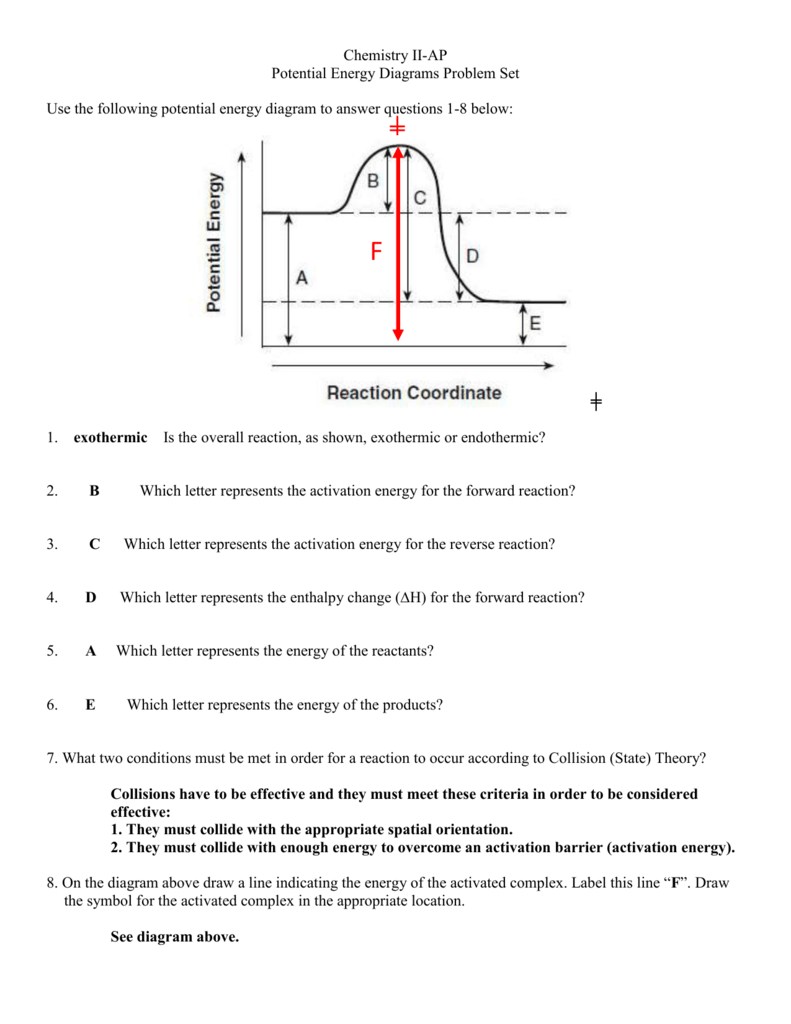
Transcribed image text: Given the following potential energy diagram for a 3 step reaction, label or identify which arrow shows these categories for both the forward and reverse reactions (do forward in one color and reverse in a different color): a.) Reactant(s) b.) Product(s) c.) Intermediate(s) d.) Rate determining step e.) ist activation energy f.) 2nd activation energy g.) The minimum amount of energy required by the reactants to undergo a reaction is known as activation energy. And when we add a catalyst into a reaction then it leads to a decrease in activation energy. As a result, product formation becomes faster. Therefore, we can conclude that intervals 1 and 3 are affected by the addition of a catalyst. 1. Identify the general shape of the energy diagram Energy should conserve for any chemical reaction. The reaction in question is exothermic (releases heat) hence its products shall have chemical potential energies lower than that of its reactants- some of the potential energies have been converted to thermal energy during the reaction process. Also, a potential energy diagram represents the activation energy as well as the heat of the reaction. Answer and Explanation: 1 The given decomposition reaction occurs in a single step.
43 Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? (1) The activation energy is 10. kJ and the reaction is endothermic. (2) The activation energy is 10. kJ and the reaction is exothermic.
Answer to: Given the potential energy diagram for a reversible chemical reaction: Each interval on the axis labeled Potential Energy (kJ/mol)...
r based upon the given potential energy diagram and indicate if the reaction endothermic or exothermic. What Is Given? You have a potential energy diagram. Plan Your Strategy Act on Your Strategy Refer to the scale on the y-axis and determine the difference in energy between the reactants and the transition state, E a(fwd); the difference in energy
The potential energy diagram for a reaction R rarr P is given byDelta H^(@) of the reaction compounds to the energy Class:11Subject: CHEMISTRYChapter: RATES ...
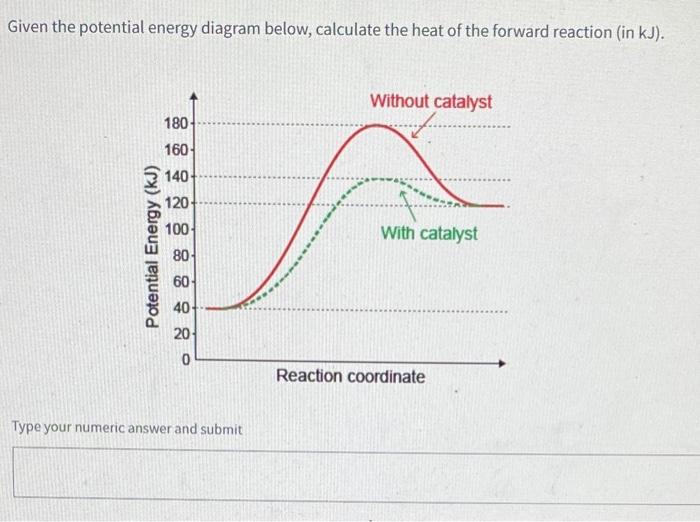
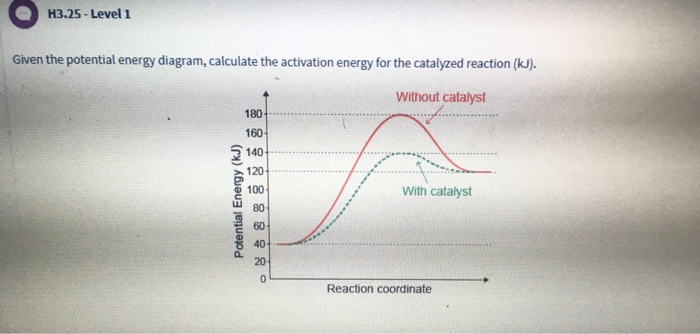
Transcribed Image Textfrom this Question. H3.25-Level 1 Given the potential energy diagram, calculate the activation energy for the catalyzed reaction (kJ). Without catalyst 1801 160 140 120 100 80 60 40 With catalyst 0. 20 Reaction coordinate.
Activation energy is defined as the least possible amount of energy (minimum) which is required to start a reaction or the amount of energy available in a chemical system for a reaction to take place →A. C → H o is the difference of the forward and backward activation energies. Hence, the correct answer is option A.
Solved Explosive Reactions Have A High Activation Energy And Explosive Substances Can Be Safely Stored For Years The Potential Energy Diagram For Course Hero
Thermodynamics Review 27. Given the potential energy diagram: With reference to energy, the reaction A + B → AB can best be described as 1) endothermic, having a +ΔH 2) endothermic, having a -ΔH 3) exothermic, having a +ΔH 4) exothermic, having a -ΔH 28.
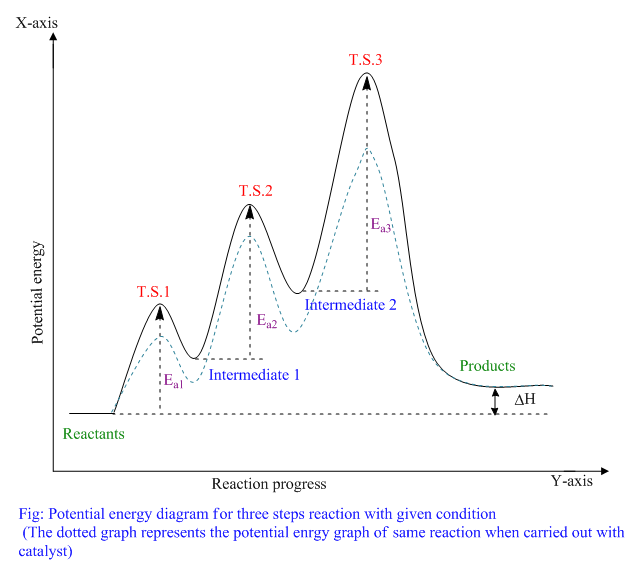
The reaction whose potential energy diagram is shown in the figure is a two-step reaction. The activation energy for each step is labeled E a1 and E a2 .Each elementary step has its own activated complex, labeled AC 1 and AC 2 .Note that the overall enthalpy change of the reaction is unaffected by the individual steps, since it depends only on the initial and final states.
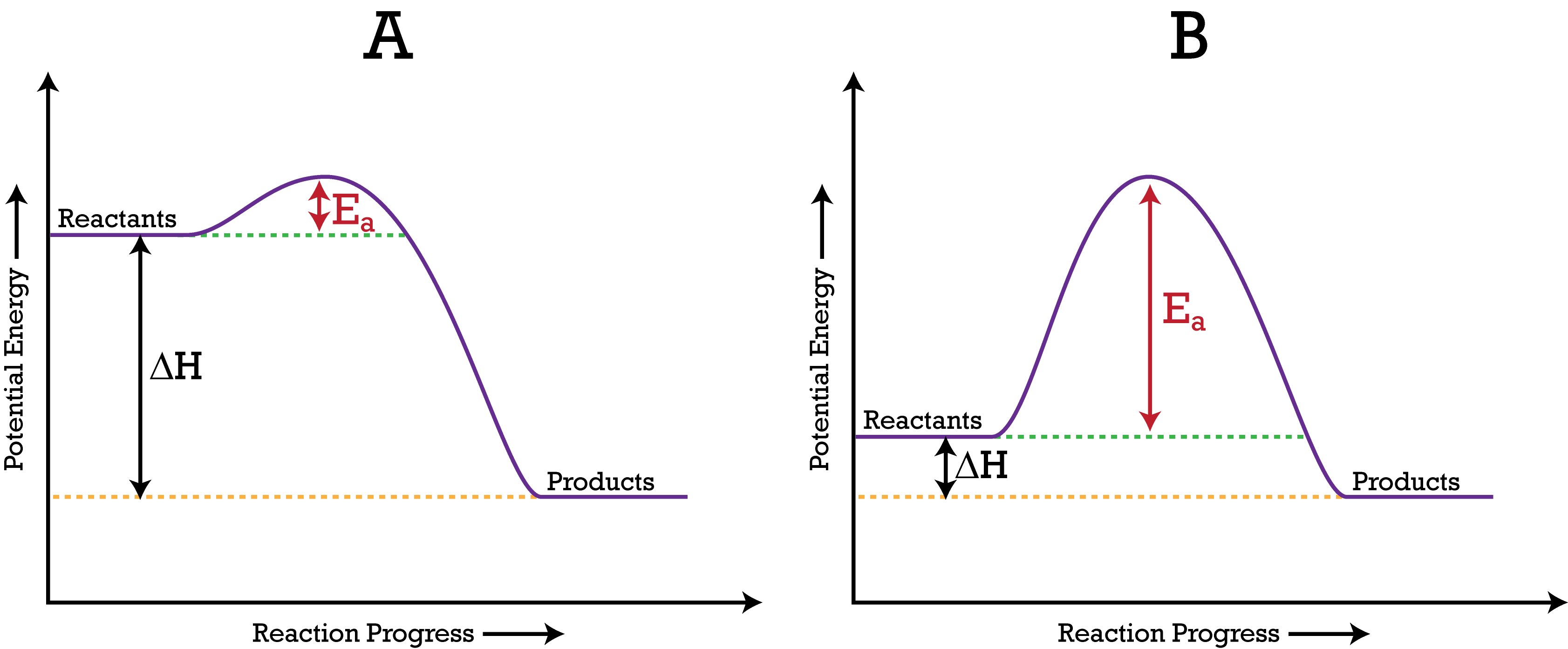
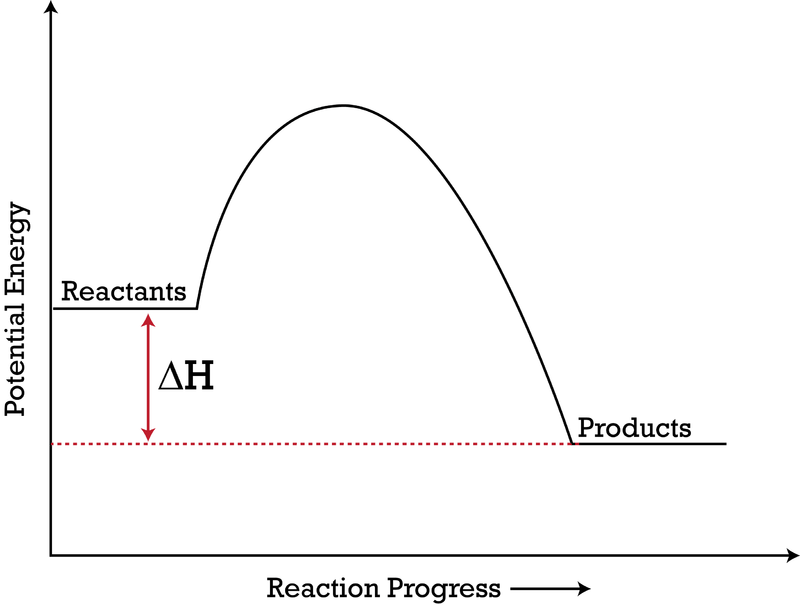
A potential energy diagram shows the change in potential energy of a system as reactants are converted into products. The figure below shows basic potential energy diagrams for an endothermic (A) and an exothermic (B) reaction. Recall that the enthalpy change is positive for an endothermic reaction and negative for an exothermic reaction. This ...
Given the potential energy diagram for a chemical reaction: Which statement correctly describes the energy changes that occur in the forward reaction? A. The activation energy is 10. kJ and the reaction is C. The activation energy is 50. kJ and the reaction is endothermic. endothermic. B.
The potential energy diagram shown below represents the reaction a + b + 50 kJ c + Reaction Coordinate Given the potential energy diagram: Reaction Coordinate Does this potential energy diagram represent an exothermic or an endothermic reaction? [Explain why.] Which lettered interval represents the potential energy of the actants of the
HELP ASAP I WILL GIVE BRAINLIST The potential energy diagram for a reaction starts at 380 kJ and ends at 100 k… Get the answers you need, now!
Which Of He Following Is Potential Energy Diagram For Sn1 Reaction Sarthaks Econnect Largest Online Education Community
11.Given the potential energy diagram for a reaction: Which intervals are affected by the addition of a catalyst? A)It is endothermic, and entropy decreases. B)It is endothermic, and entropy increases. C)It is exothermic, and entropy decreases. D)It is exothermic, and entropy increases. 12.Given the balanced equation representing a phase change:
The reaction NO2 (g) + CO (g) → NO (g) + CO2 (g) has ΔH°overall = -226 kJ/mol. A proposed reaction mechanism is shown. Choose the statement (s) that accurately describe the reaction energy diagram for the above reaction. -There will be three peaks. -The Ea of the first step will be larger than the second or third step.
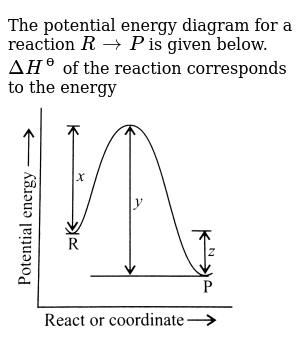
The Potential Energy Diagram For A Reaction R Rarr P Is Given Below Delta H ɵ Of The Reaction Corresponds To The Energy Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images Ksv Phy Chm P2 C04 E01 195 Q01 Png Width 80
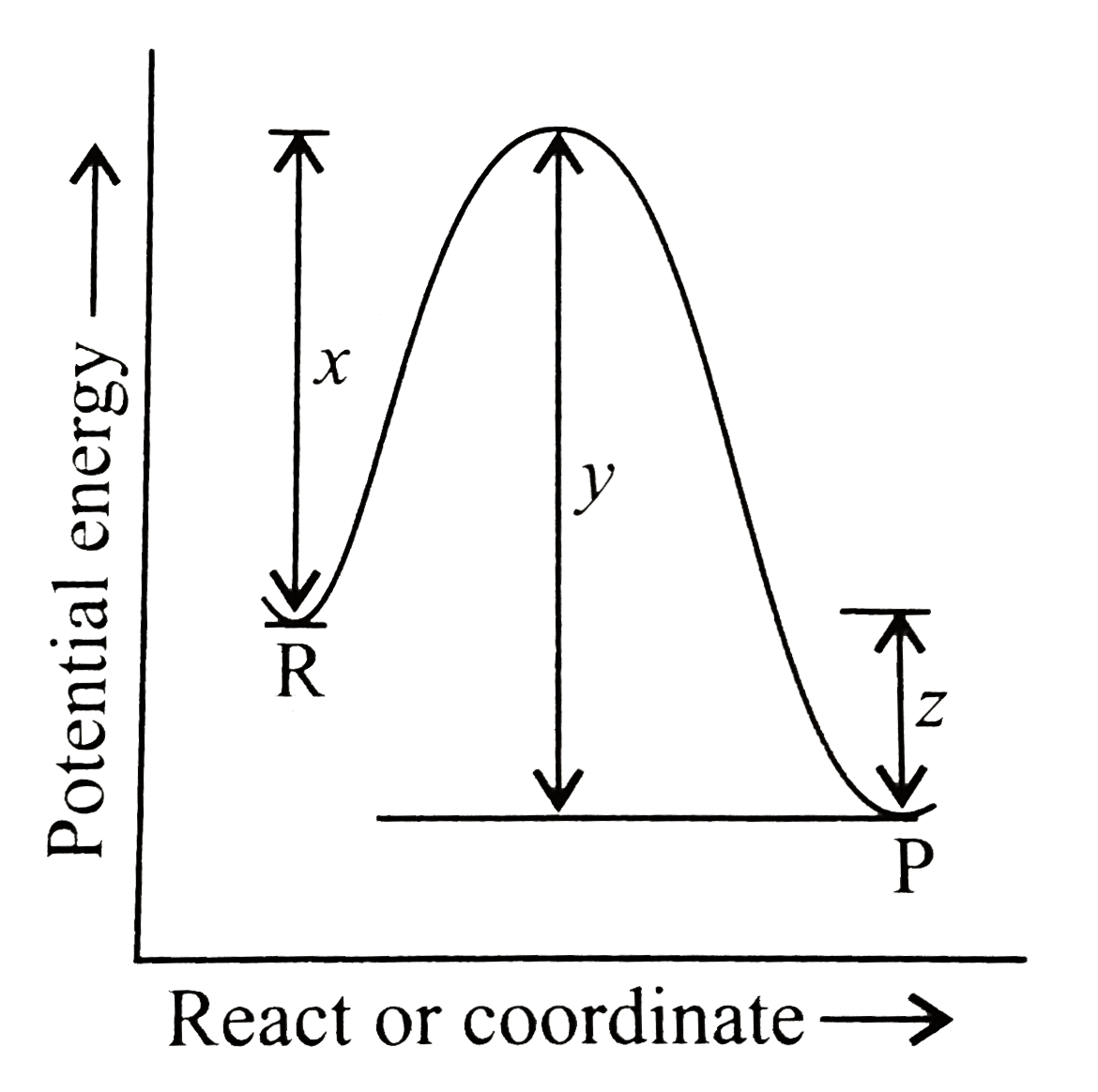
The Potential Energy Diagram For A Reaction R Rarr P Is Given Below Delta H ɵ Of The Reaction Corresponds To The Energy Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images Ksv Phy Chm P2 C04 E01 195 Q01 Png Width 80
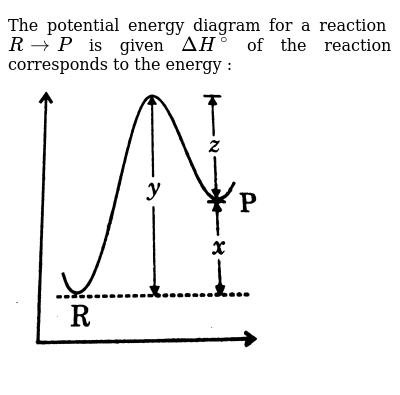
The Potential Energy Diagram For A Reaction R Rarr P Is Given Deltah Of The Reaction Corresponds To The Energy Img Src Https D10lpgp6xz60nq Cloudfront Net Physics Images Grb Phy Chm P2 V03 Qb C13 E01 207 Q01 Png Width 80

The Potential Energy Diagram For A Reaction R Rarr P Is Given Below Delta H ɵ Of The Reaction Youtube
















0 Response to "44 given the potential energy diagram for a reaction"
Post a Comment