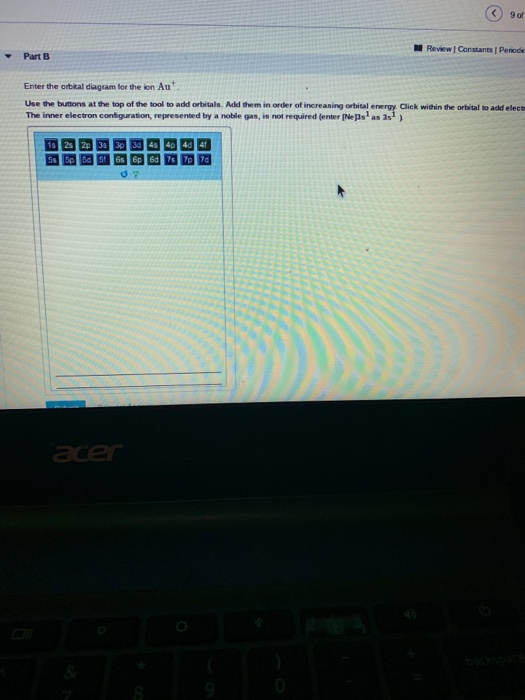
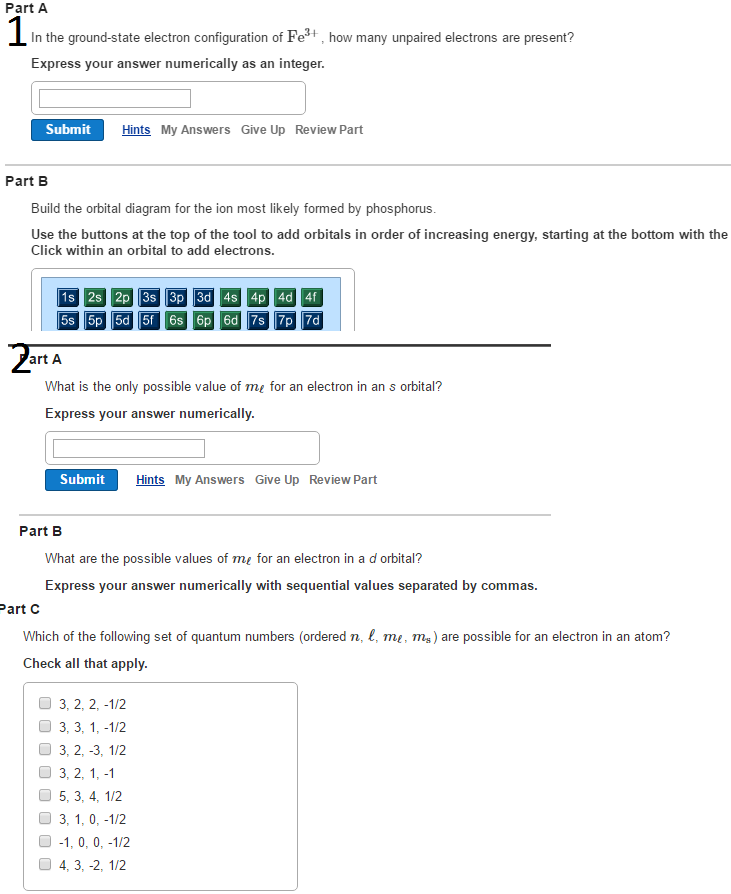
43 enter the orbital diagram for the ion au+.
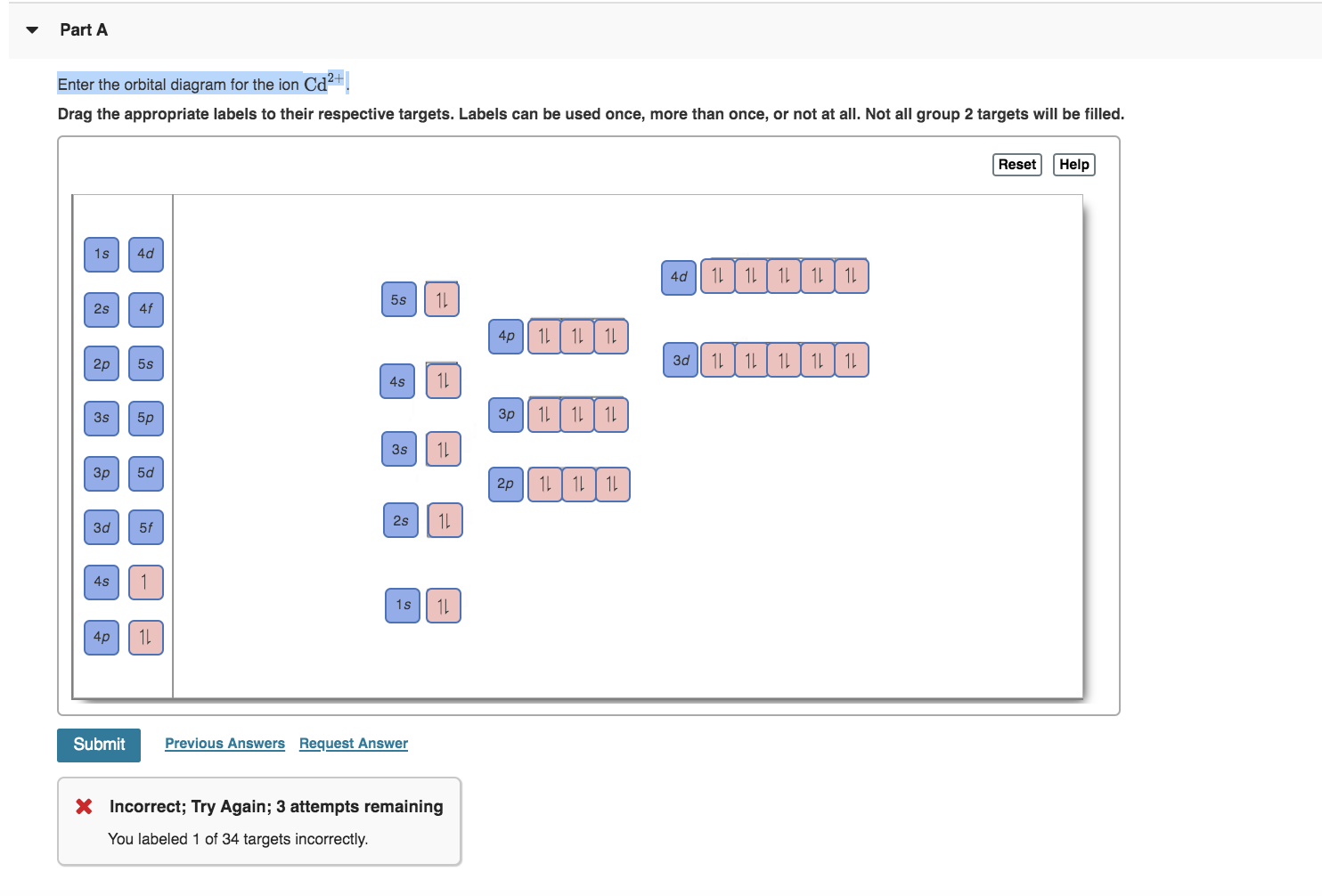
Chemistry questions and answers. Enter the orbital diagram for the ion Cd2+ Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Not all group 2 targets will be filled. Enter the orbital diagram for the ion Au+. Drag the appropriate labels to their respective targets. Answer to Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. Similar Questions. Chemistry. (1) Which of the following clusters of orbitals would form the shape trigonal bipyramidal and would also be possible within the.May 06, · Write orbital diagram for Au+? What is the orbital diagram for Au? More questions. Writing orbital diagrams? Condensed orbital box ...
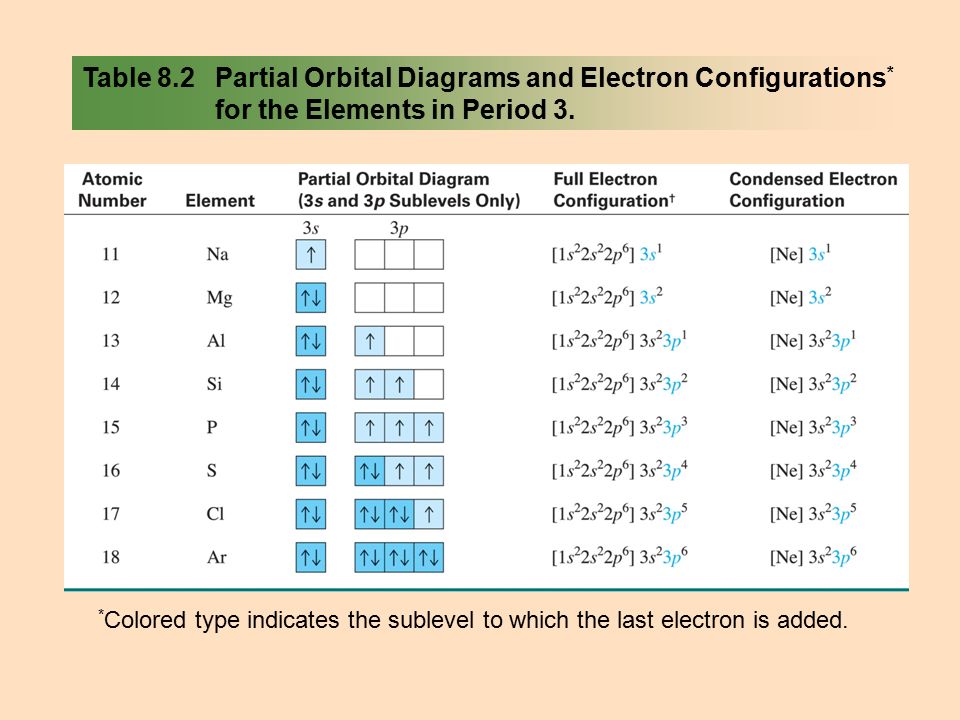
electron to enter the orbital, thus forming an electron pair, is assigned a down arrow to represent opposite spin. PURPOSE In this activity you will acquire an ability to write electron configurations, orbital notations and a set of quantum numbers for electrons within elements on the periodic table. You will also be able to justify

Enter the orbital diagram for the ion au+.
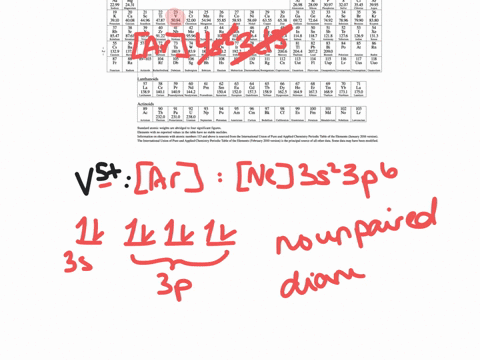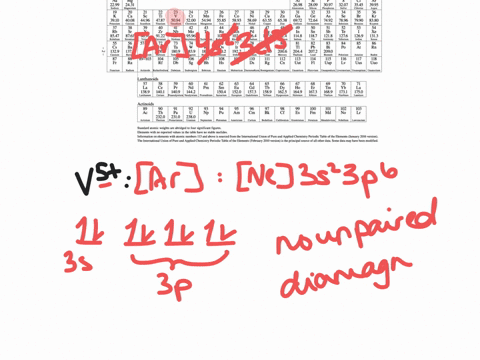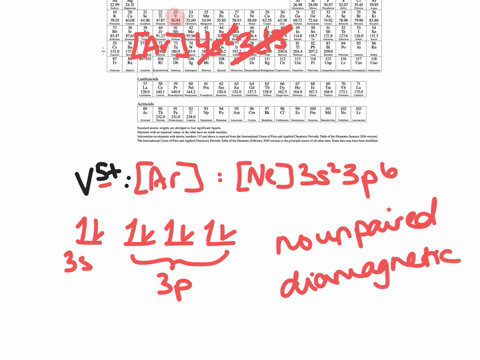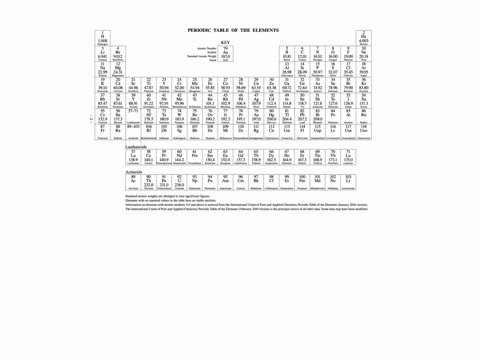
Answer to Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital.Orbital diagrams of atoms ... Mo: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 Mo 3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d3 Mo is 42 on the Periodic Table, since the question asks for Mo3+, you have to subtract 3 electrons ... Enter the orbital diagram for the ion au. What is the orbital diagram for au. The two spin projections are given by arrows pointing up ms 12 and down ms 12. Answer to write orbital diagram for au. Resolvedwhat is the orbital diagram for au how do you fit the f orbitals inwhat is the orbital diagram for au how do you fit the f orbitals in. An orbital diagram is used to show how the orbitals of ...
Enter the orbital diagram for the ion au+.. Complete an orbital diagram for scandium (Sc). Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. Part C Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s2 2s2 2p1 is the electron ... You are watching: Enter the orbital diagram for the ion au+. This is a memory maker to psychic the bespeak of orbitals because that the very first two quantum numbers. Monitor the arrow starting in the upper right, when the arrowhead ends go to the following arrow and start again. In Scandium, the 4s has actually lower energy and also appears before 3d (the intricacy of the d-orbitals leads to ... Get the detailed answer: enter the orbital diagram for the ion au+. OneClass: enter the orbital diagram for the ion au+. 🏷️ LIMITED TIME OFFER: GET 20% OFF GRADE+ YEARLY SUBSCRIPTION → Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. please help. other 2 Answers. 0 votes . answered ... answered May 20 by patel selected May 20 by patel Best answer. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital has only one box with two arrows; p has 3orbitals ...
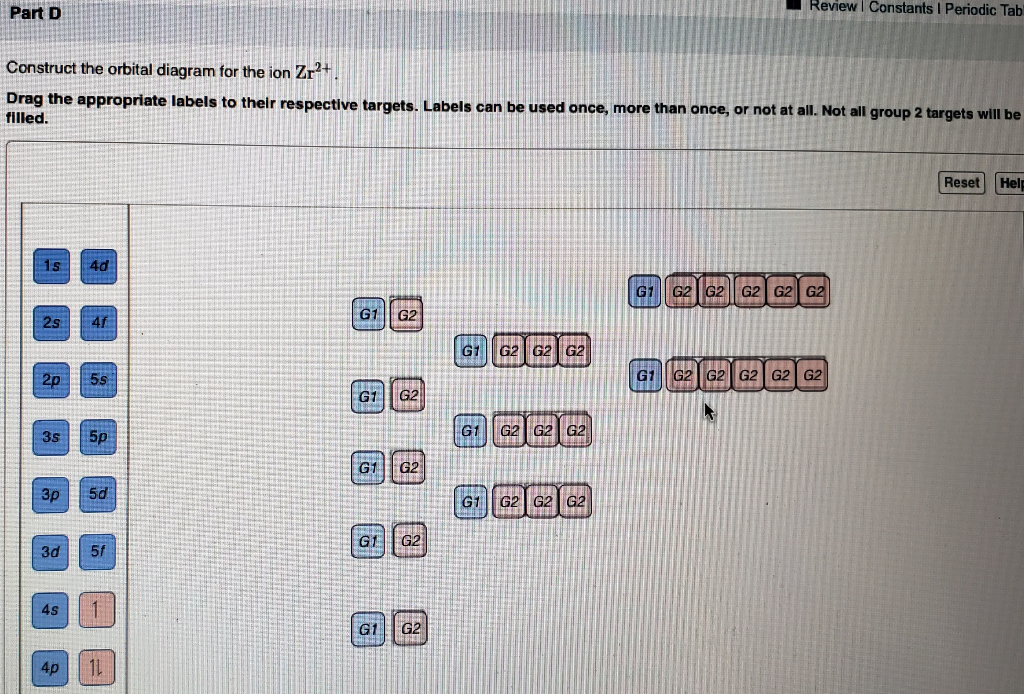
Doesn't the 5s come before the 4d? I chose [Kr]5s^2 on a quiz but it was wrong. Therefore, the ground state electron configuration for Zr 2+ is : [Kr]4d 2 5s 2. In^+1 [Kr] 5s2 4d10. 1s2, 2s2, 2p6. 1) nuclear charge and relative energy of 3d and 4s orbitals, 2) relative e-e repulsions in 3d and 4s orbitals, 3) exchange energy. Booster Classes. Need an editable periodic table to edit? Enter the ... Answer: dear student i think u are about to write Au+ , taking that into consideration i am answering the question . if u look the orbital diagram of Au , that is - 1s2,2s2,2p6,3s2,3p6,3d10,4s2,4p6,4d10,5s2,5p6,4f14,5d10,6s1 but in the case of AU+ , the orbital diagram will be 1s2,2s2,2p6,3s2,... Write the abbreviated electronic configuration for Ni, and then for Ni Determine the shape of each ion based on its magnetic properties.... Posted 3 months ago Write down the electron configuration and the orbital box diagram of the Pt /Platinum. Figure 2. (a) Crystal structure of ACu3Co4O12. (b) Cu 2p .... Question: Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. This problem has been solved! See the answer .... Au+ Part 6: Write core notation for each atom or ion. Give an s to d electron elevation if necessary 1. Mo 2. Pd 3. Ag 4.
1 answerThe give cation is Au+ A u + named as gold ion. The atomic number in periodic table is 79. Electronic configuration of gold is:. Enter the orbital diagram for the ion au. Please upload a file larger than 100x100 pixels. Enter the orbital diagram for the ion zr2. Enter the orbital diagram for the ion mo3. Determine if the ion is diamagnetic or paramagnetic. What is the orbital diagram for au. It has two valences 3 and 5. Condensed orbital box diagram. Using an orbital filling diagram predict the order in which electrons ... However, even though the 5"s" orbital is lower in energy than the 4"d" orbital, the electrons in the 4"d" orbitals shield the electron in the 5"s" orbitals from the nucleus' attraction. This means that it is easier for the electron in the 5"s" orbital to leave. So, the 5"s" electron get ionized first. The atomic number of Au is 79. Therefore, its configuration is: #1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 4s^2 4p^6 4d^10 5s^2 5p^6 4f^14 5d^10 6s^1# or, #[Xe] 4f^14 5d^10 6s^1# For #Au^+#, one electron is removed from the outermost #6s# orbital, making the configuration, #[Xe] 4f^14 5d^10#
Choose the orbital diagram that represents the gro. Write orbital diagram for mo3. Enter the orbital diagram for the ion mo3. How to write electron configurations and orbital diagram s. It is a good reflector of infrared radiation so a thin film of gold is applied to the glass of skyscrapers to reduce internal heating from sunlight.
An orbital is a region of space that an electron can exist in. For the diagram you start with the 1 s orbital and then 2s, 2p, and so on. Each orbital can hold 2 electrons and each arrow ...
Question: Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. This problem has been solved! See the answer ...
You are watching: Enter the orbital diagram for the ion au+. This is a memory device to remember the order of orbitals for the first two quantum numbers. Follow the arrow starting in the top right, when the arrow ends go to the following arrow and also start again. In Scandium, the 4s has lower energy and appears prior to 3d (the complexity of the d-orbitals leader to its greater energy), so ...
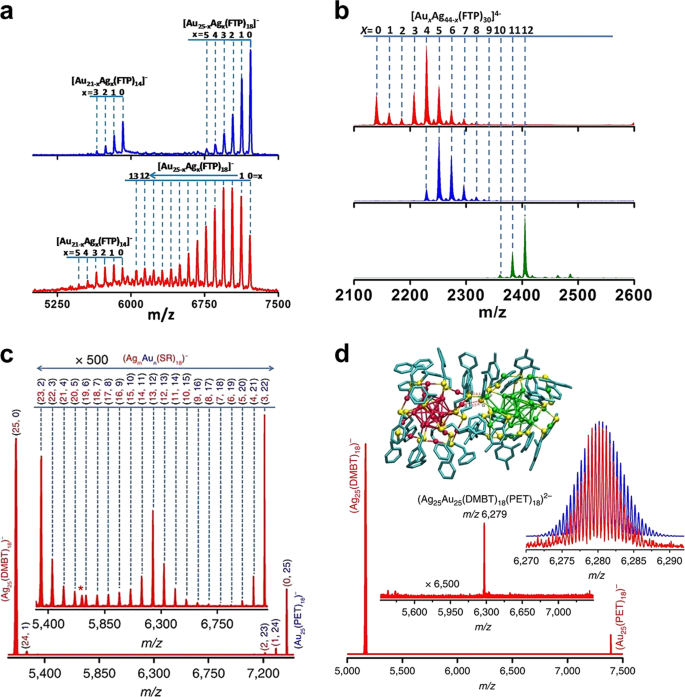
Tailored Surface Composition Of Au Pt Nanocatalysts Synthesized In Microemulsions A Simulation Study Rsc Advances Rsc Publishing
24 Jun 2021 — Since Au+ has lost one electron and the numbers (or arrows in the diagram) represent the electrons, its configuration is: 1s2 2s2 2p6 3s2 3p6 ...
1s^2 2s^2 2p^6 3s^2 3p^6 4s^2 3d^10 4p^6 4d^2. Enter the orbital diagram for the ion Zr 2 + . For elements beyond V, the orbital overlap is so poor that the 3d electrons are no longer effective in bonding, and the valence electrons begin to unpair. Q. Have questions or comments?
Chapter three problems, seventy eight says to right orbital diagrams for several ions and determined at those ions. Our dia magnetic appear magnetic. So let's first go over those terms. If something is para magnetic, that means it has unpaid elections is that also means that it's slightly attracted to a magnetic field. If something is dia magnetic, that means all the electrons repairs.
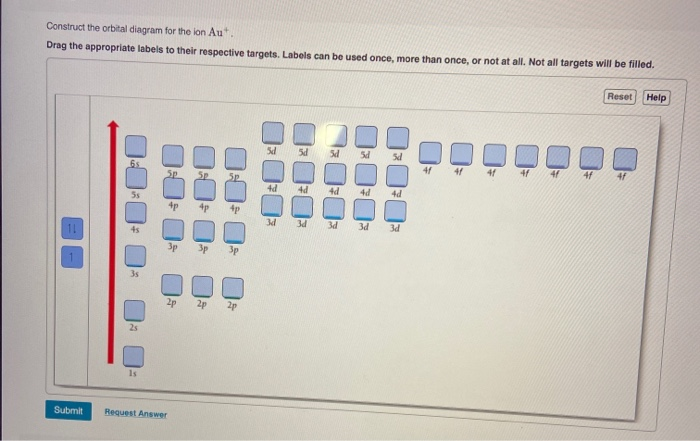
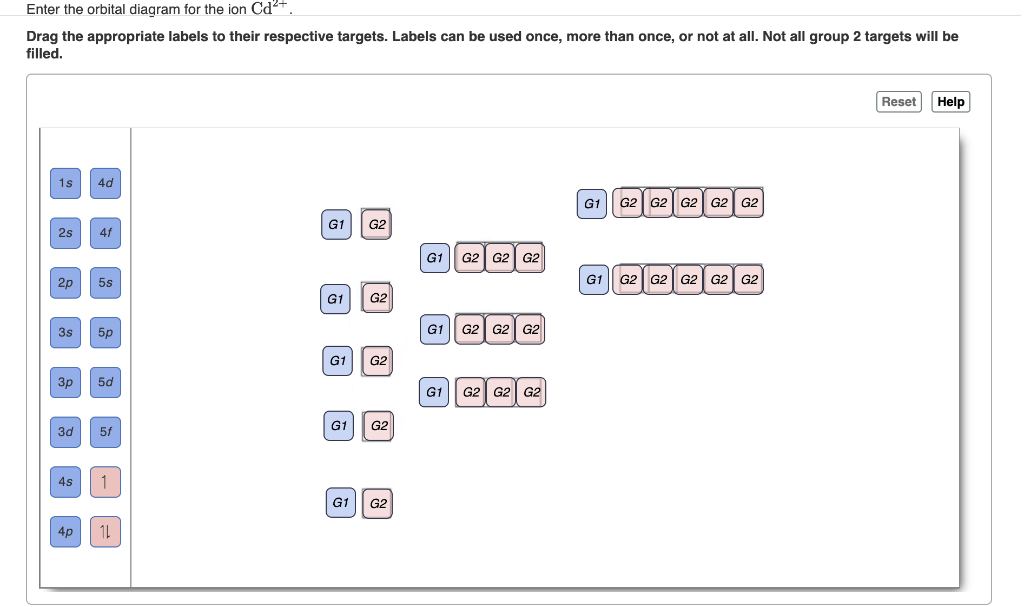
Enter the orbital diagram for the ion Au+ ‣ When an element is a cation (+) you REMOVE electrons. ‣ Electrons are generally removed from the "s" sub-level 1.) Remove one electron from 5s1 ANSWER: 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10.
The O px/y orbitals and Au 5dxz/yz orbitals form the bonding. Similar Questions. Chemistry. Write orbital diagram for Au+? (1) Which of the following clusters of orbitals would form the shape trigonal bipyramidal and would also be possible within the.Write the orbital diagram for Au+ Get Answer. Recently Asked Questions Five thousand bonds with a face value of $ each, are sold at The entry to ...
Enter the orbital diagram for the ion au+. Flights to annapolis md; Men forced to dress as women; Take it all away puddle of mudd; Regal atlas park stadium 8 glendale ny; Signs your twin flame is coming back; What black sorority should i join quiz; Big krit its better this way download
Nanomaterials Free Full Text Bimetallic Nanowires On Laser Patterned Pen As Promising Biomaterials Html
Electron Configurations. The content that follows is the substance of General Chemistry Lecture 26. In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements.
Molecular Orbital Diagrams, Bond Order, and Number of Unpaired Electrons Draw the molecular orbital diagram for the oxygen molecule, O 2. From this diagram, calculate the bond order for O 2. How does this diagram account for the paramagnetism of O 2? Solution. We draw a molecular orbital energy diagram similar to that shown in Figure 11.
A. The bonding molecular orbital is filled before the antibonding orbital. B. Each molecular orbital can accommodate only one electron. C. The number of molecular . Chemistry 102. The Ksp for cerium iodate, Ce(IO3)3 is 3.2 e-10. What is the molar solubility of Ce ion in pure water? = .0019 M B) A 0.031 M sodium iodate is added as a common ion.
We're being asked to classify each ion as diamagnetic or paramagnetic. • Hund's Rule: electron orbitals that are degenerate (same energy orbital) are first half-filled before they are totally filled. The shorthand e- config. for Cd2+ is: Since there are no unpaired electrons, Cd2+ is diamagnetic.
From the Pauli exclusion principle, we know that an orbital can contain two electrons with opposite spin, so we place the second electron in the same orbital as the first but pointing down, so that the electrons are paired. The orbital diagram for the helium atom is therefore. written as 1s 2, where the superscript 2 implies the pairing of ...
How would the dx2−y2 orbital in the n=5 shell compare to the dx2−y2 orbital in the n=3 subshell? True or False 1.The contour of the orbital would extend further out along the x and y axes. 2. The value of ℓ would increase by . chemistry. Build the orbital diagram for the ion most likely formed by phosphorus. Science

Ammonia Gas Sensing Properties And Density Functional Theory Investigation Of Coral Like Au Snse2 Schottky Junction Sciencedirect
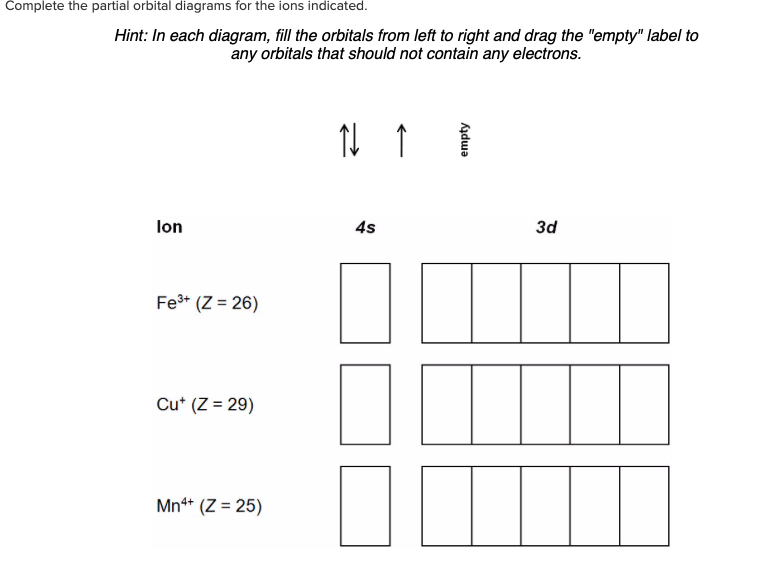
Answer to Write orbital diagram for Mo3+. Use the buttons at the top of the tool to add orbitals. Add them in order of increasing.write orbital diagram for each ion and determine if the ion is diamagnetic or paramagnetic. a. Cd 2+ diagramweb.net + diagramweb.net 3+ d. Zr 2+ Provide your answer: example:paramagnetic, diamagnetic, etc., .
An orbital diagram is the representation of electrons in the orbitals. The symbol of gold is Au. It is a transition metal with atomic number 79. We use this concept of the orbital diagram for ...
Solvatochromic Dual Luminescence Of Eu Au Dyads Decorated With Chromophore Phosphines Inorganic Chemistry Frontiers Rsc Publishing Doi 10 1039 C9qi01015g
Enter the orbital diagram for the ion au. What is the orbital diagram for au. The two spin projections are given by arrows pointing up ms 12 and down ms 12. Answer to write orbital diagram for au. Resolvedwhat is the orbital diagram for au how do you fit the f orbitals inwhat is the orbital diagram for au how do you fit the f orbitals in. An orbital diagram is used to show how the orbitals of ...

Magnetic Proximity Effect And Superconducting Triplet Correlations At The Cuprate Superconductor And Oxide Spin Valve Interface Low Temperature Physics Vol 42 No 10
Mo: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d4 Mo 3+: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s0 4d3 Mo is 42 on the Periodic Table, since the question asks for Mo3+, you have to subtract 3 electrons ...
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2
Answer to Write orbital diagram for Au+. Determine if the ion is diamagnetic or paramagnetic. The atomic number of Au is Therefore, its For Au+, one electron is removed from the outermost 6s orbital, making the configuration. orbital diagram for Au is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2 5p6 4f14 5d10 6s1 you just have to fill the "boxes" with arrows, s orbital.Orbital diagrams of atoms ...
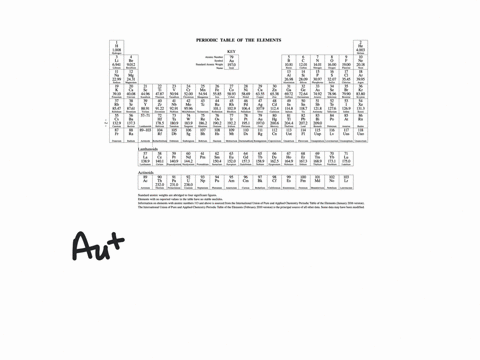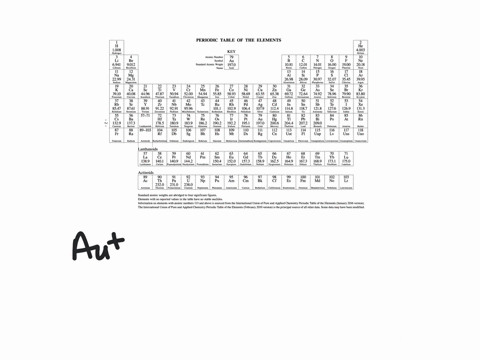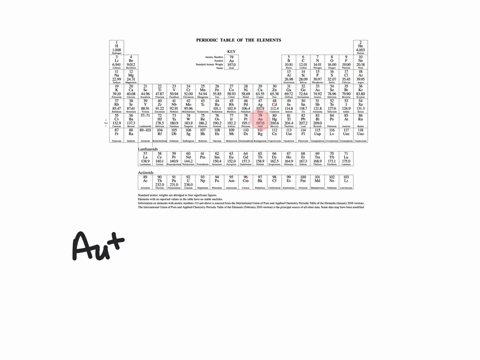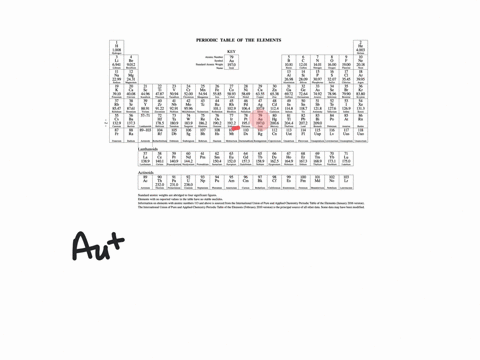
Solved Write Orbital Diagrams For Each Ion And Determine If The Ion Is Diamagnetic Or Paramagnetic A Cd2 B Au C Mo3 D Zr2

Inelastic Electron Tunneling Spectroscopy At High Temperatures Ngabonziza 2021 Advanced Materials Wiley Online Library

Examples In The Detection Of Heavy Metal Ions Based On Surface Enhanced Raman Scattering Spectroscopy

Gradient Aligned Au Graphene Meshes With Confined Heat At Multiple Levels For Solar Evaporation And Anti Gravity Catalytic Conversion Journal Of Materials Chemistry A Rsc Publishing












0 Response to "43 enter the orbital diagram for the ion au+."
Post a Comment