43 calculating the wavelength of a spectral line from an energy diagram
After you obtain the energy, then you can realize that that energy has to correspond exactly to the energy of the photon that came in: #|DeltaE| = E_"photon" = hnu = (hc)/lambda# where #h# is Planck's constant, #c# is the speed of light, and #lambda# is the wavelength of the incoming photon. Thus, the wavelength is: Steps for Calculating Wavelength of a Spectral Line from an Energy Diagram. Step 1: Determine the energy of transition of interest. Step 2: Convert the energy into a wavelength. Step 3: Convert ...
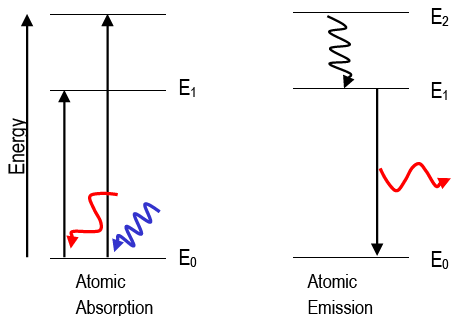
Energy level diagrams are a means of analyzing the energies electrons can accept and release as they transition from one accepted orbital to another. These energies differences correspond to the wavelengths of light in the discreet spectral lines emitted by an atom as it goes through de-excitation or by the wavelengths absorbed in an absorption ...
Calculating the wavelength of a spectral line from an energy diagram
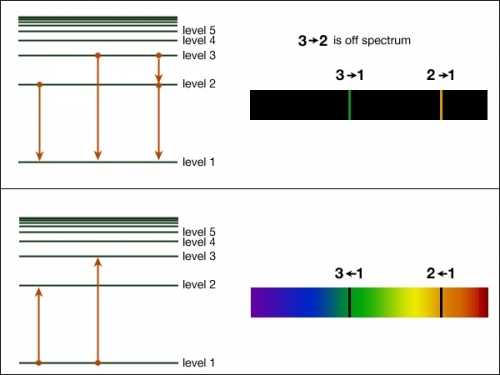
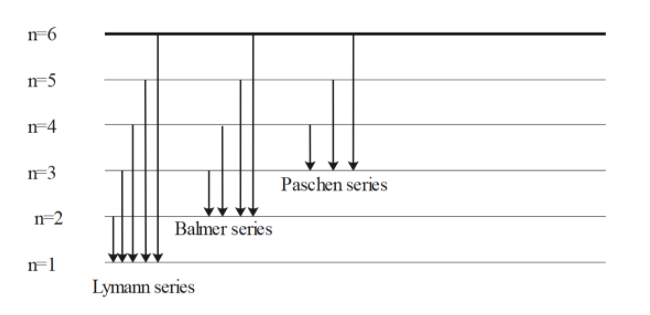
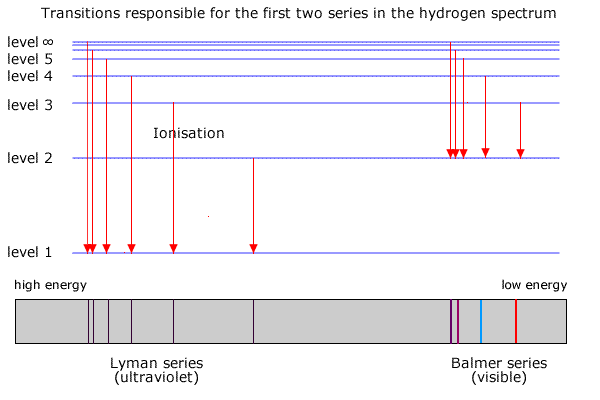
Spectral lines arise as a result of electronic transitions in atoms. The Balmer series is produced by the transitions shown in the following diagram. (a) What transition corresponds to a line A in the spectrum? Explain your answer. 2 (b) Calculate the energy difference, in kJ mol-1, that gives rise to line A, with wavelength 656 nm. 3 SY/91 ... (a) Draw the energy level diagram for the line spectra representing Lyman series and Balmer series in the spectrum of hydrogen atom. (b) Using the Rydberg formula for the spectrum of hydrogen atom, calculate the largest and shortest wavelengths of the emission lines of the Balmer series in the spectrum of hydrogen atom. (Use the value of Rydberg constant ) The diagram below shows the line spectrum of a gas. € 1 Explain how line spectra are produced. In your answer you should describe: •€€€€€€€€how the collisions of charged particles with gas atoms can cause the atoms to emit photons. •€€€€€€€€how spectral lines are explained by the concept of discrete energy ...
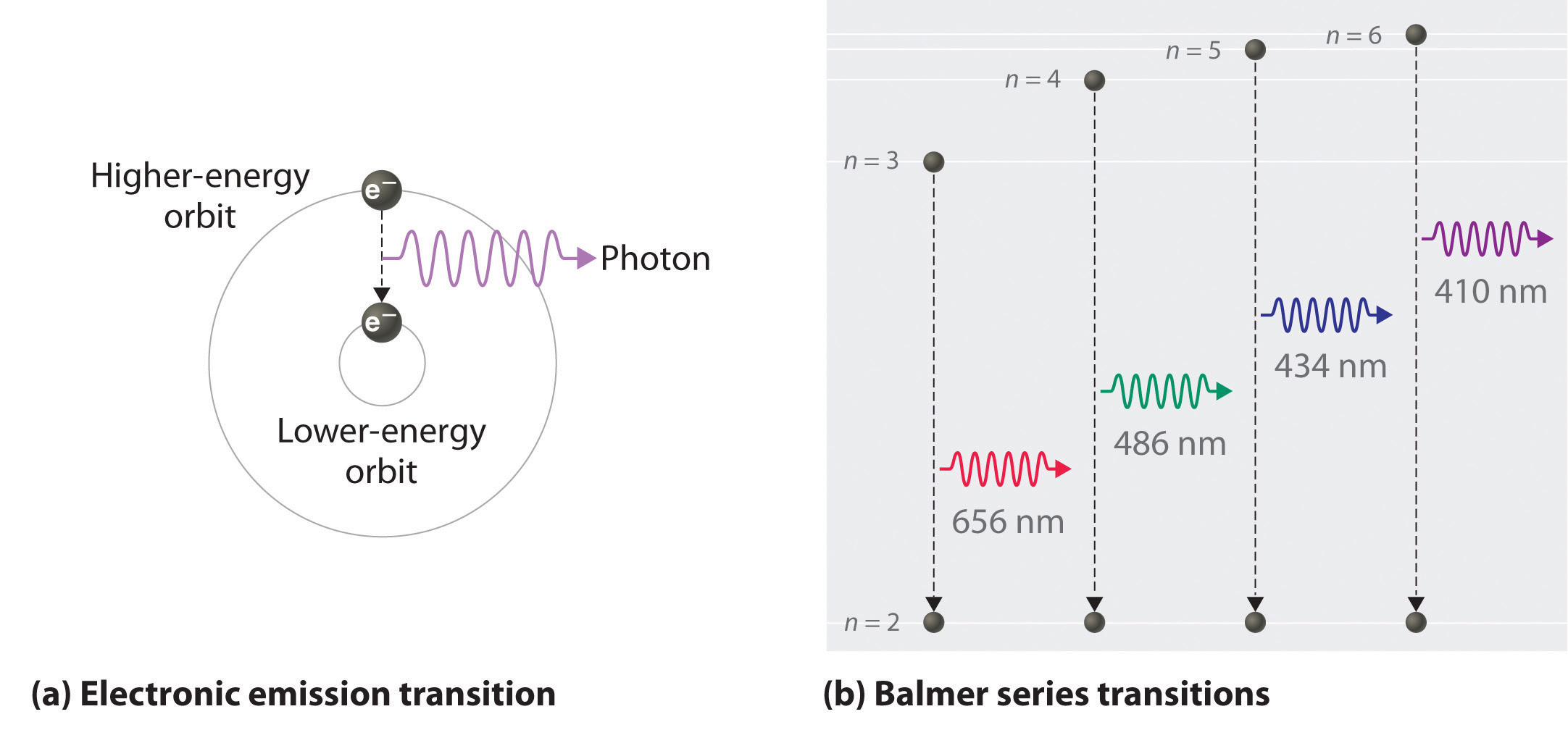
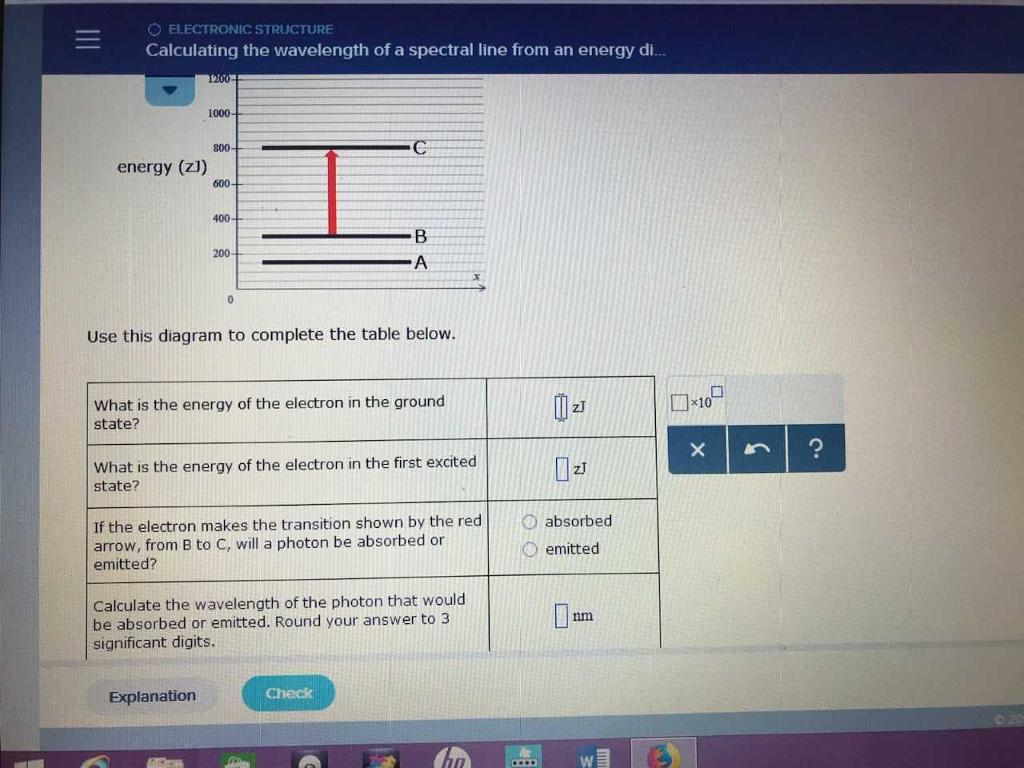
Calculating the wavelength of a spectral line from an energy diagram. 6.4, we see that the wavelength of this electron is about the same as that of X -rays. Solution. Analyze: We are given the mass, m, and velocity, v, of the electron, and we must calculate its de Broglie wavelength, λ. Plan: The wavelength of a moving particle is given by Equation 6.8, so is calculated by inserting the known quantities . h, m ... Calculate the wavelength of the spectral line, when the electron in the hydrogen atom undergoes a transition from the energy level 4 to energy level 2. According to the Bohr model, the wavelength of the light emitted by a hydrogen atom when the electron falls from a high energy (n = 4) orbit into a lower energy (n = 2) orbit.Substituting the appropriate values of R H, n 1, and n 2 into the equation shown above gives the following result.. Solving for the wavelength of this light gives a value of 486.3 nm, which agrees with the experimental ... Calculating the wavelength of a spectral line from an energy diagram The lowest possible energy is called the ground state, all other states are called excited states • A has the lowest energy at 350 zJ • B is next at 500 zJ An electron making a transition to a state of lower energy will emit a photon to get rid of excess energy An electron going to a higher state will.
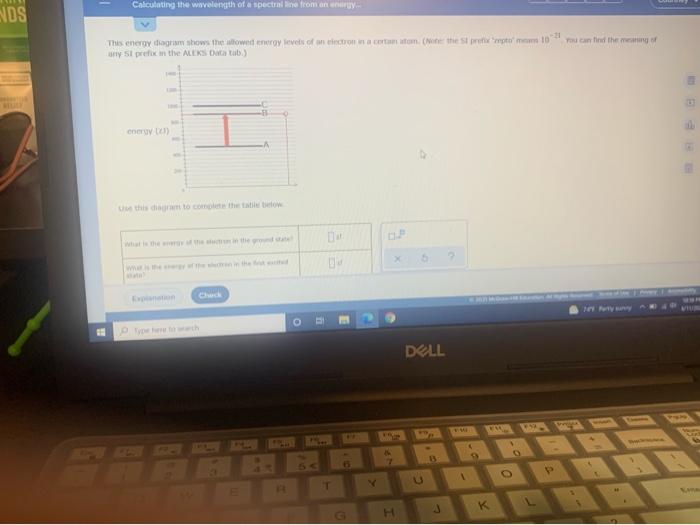
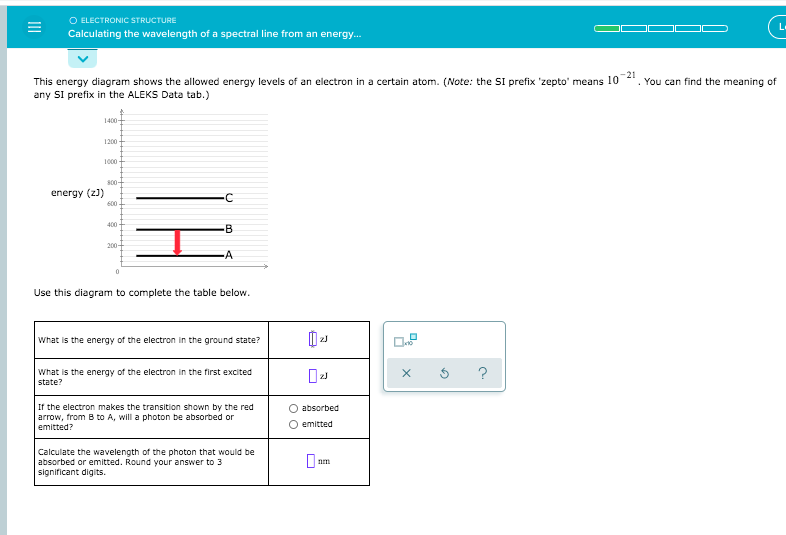
Transcribed image text: O ELECTRONIC STRUCTURE Calculating the wavelength of a spectral line from an energy. You can find the meaning of This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the Si prefix zepto' means 102 any Si prefix in the ALEKS Data tab.) 1 C 200 energy (23) M 4 Use this diagram to complete the table below What is the energy of the ... The diagram below shows the energy level diagram of a hydrogen atom. The associated spectrum to the diagram above is shown below. The transition labelled A in the top diagram gives the spectral line labelled B in the spectrum diagram. (a) (i) Show that the frequency of spectral line B is about 4.6 × 10 14 Hz. ΔE = 3.40-1.51 = 1.89 eV. Electron Transitions The Bohr model for an electron transition in hydrogen between quantized energy levels with different quantum numbers n yields a photon by emission with quantum energy: This is often expressed in terms of the inverse wavelength or "wave number" as follows: The reason for the variation of R is that for hydrogen the mass of the orbiting electron is not negligible compared to ... Transcribed image text: O ELECTRONIC STRUCTURE AND CHEMICAL BONDING Calculating the wavelength of a spectral line from his energy diagram shows the allowed energy levels of ny SI prefix in the ALEKS Data tab.) 1400- 1200 1000 -C 800 energy (2) 600 В 400 200 -A Use this diagram to complete the table below.
Calculate the wavelength of the photo that would be absorted Initted. Round your answer to significant Explanation Check This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: t ny SI prefix in the ALEKS Data tab.) 1400 1200 1000 - c 800 energy (23) 600 DU 400 200 0 Use this diagram to complete the table below. where RH is the Rydberg constant, Z is the atomic number, and λ is the wavelength of light emitted, could be explained by the energy differences between the quantized electron energies n.Since the Bohr model applies to hydrogen-like atoms, i.e., single-electron atoms, for the case of He+, Z=2 and RHZ2 = 4.38949264 x 107 m-1.We can use this equation to calculate the ionization potential of He+ ... Calculate the wavelength, in nanometers, of the spectral line produced when an electron in a hydrogen atom undergoes the transition from the energy level 6 to the level n = 1. The ground state energy of hydrogen atom is − 13.6 eV. If an electron makes a transition from an energy level − 1.51 eV to − 3.4 eV, then calculate the wavelength of the spectral line emitted and name the series of hydrogen spectrum to which it belongs.
Solved O Licironic Structu Critulating The Wavelength Of A Spectral Line From On Energy This Energy Diagram Shows The Allowed Energy Levels Of On Course Hero
Since the difference in energy between states B and A is , the photon emitted during the transition must have an energy of . To calculate the wavelength of the photon, you'll need to use the relationship between photon energy andwavelength for electromagnetic radiation:350zJ 700zJ =−700.zJ 350.zJ 350.zJ 350. zJ.
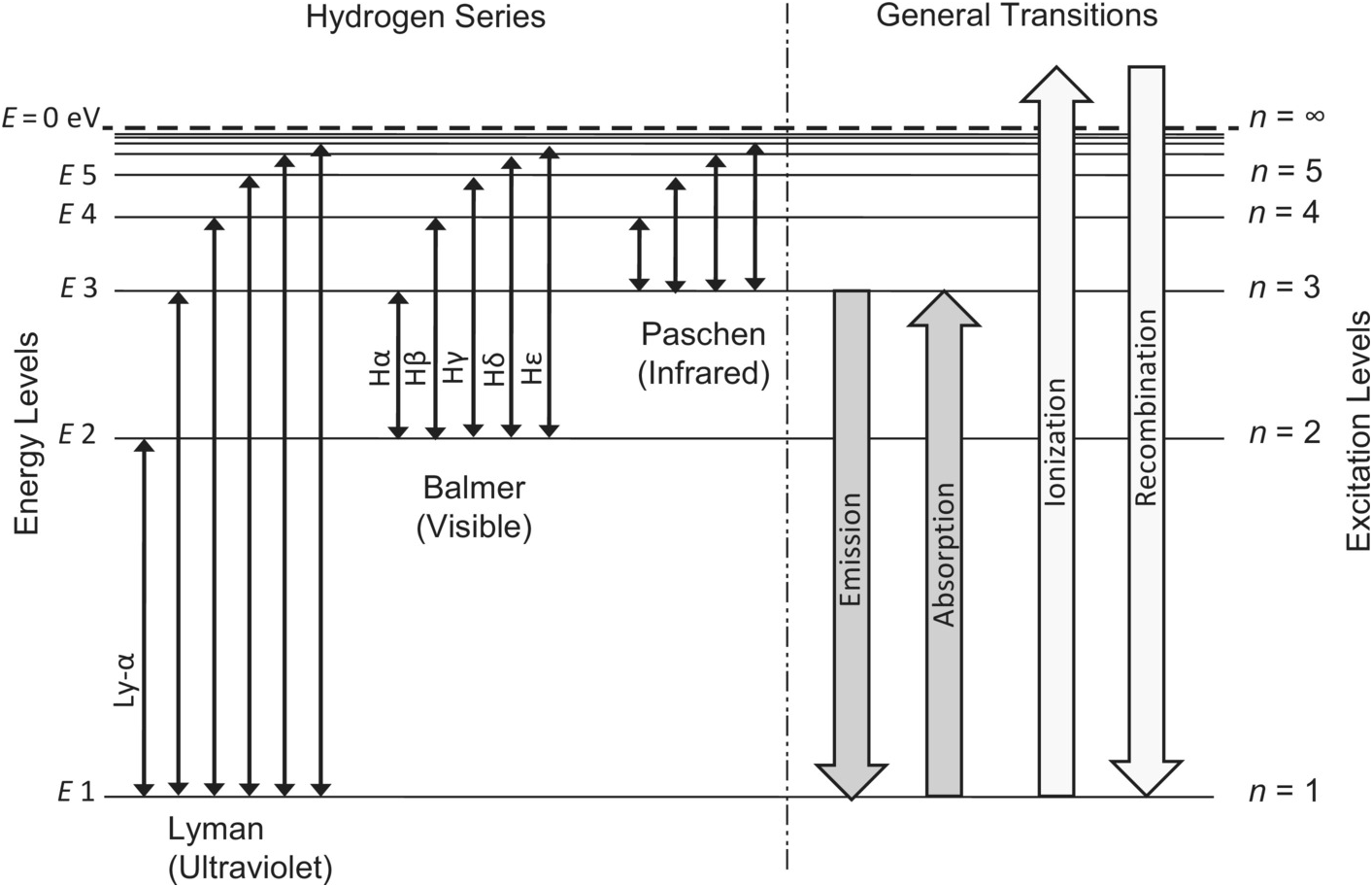
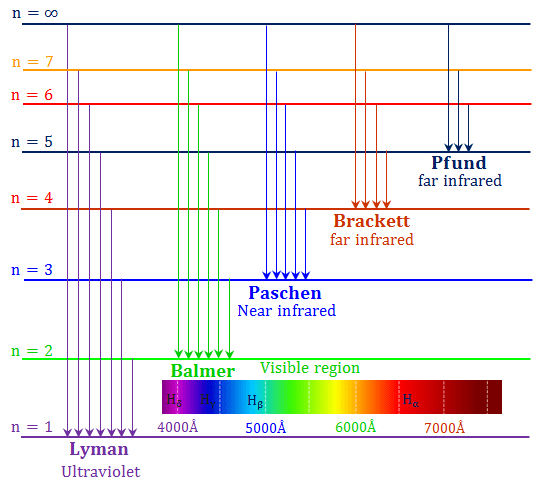
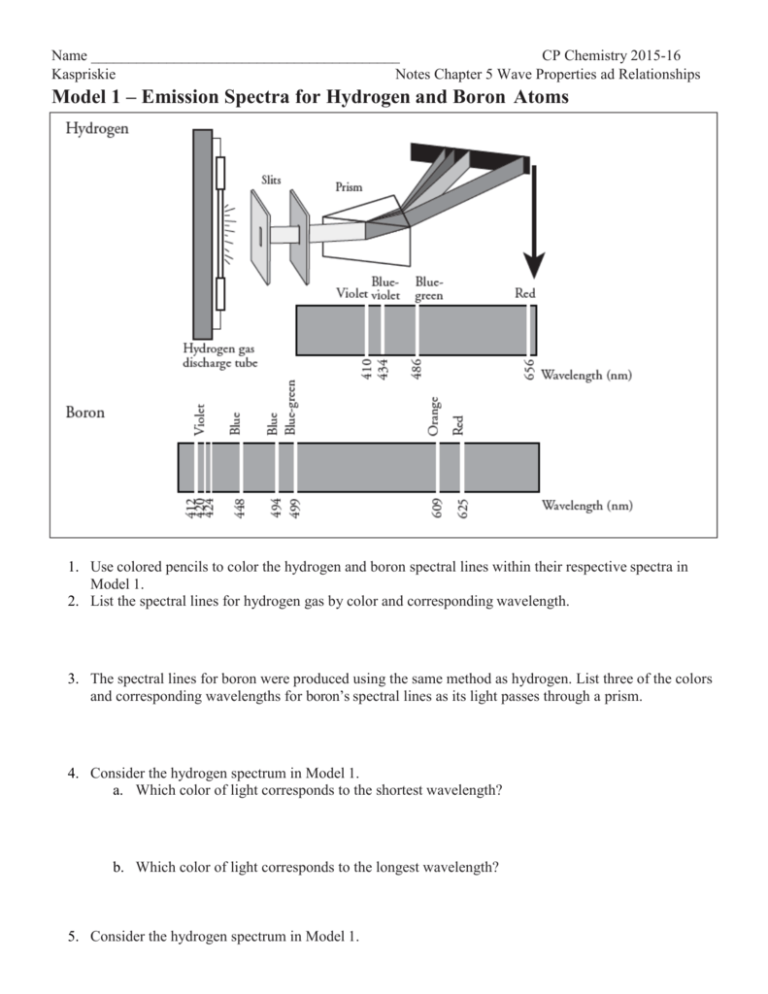
Jahann Balmer in 1885 derived an equation to calculate the visible wavelengths that the hydrogen spectrum displayed. The lines that appear at 410 nm, 434 nm, 486 nm, and 656 nm. These electrons are falling to the 2nd energy level from higher ones. This transition to the 2nd energy level is now referred to as the "Balmer Series" of electron ...
Draw the energy level diagram for the line spectra representing Lyman Series and Balmer series in the spectrum of Hydrogen atoms. Answer. Verified. 98.1k+ views. Hint: When an electron makes a transition from one orbit to the other in an atom, it gains or loses energy due to which it emits wavelengths corresponding to the energy differences ...
It is called a spectral line. As the wavelength of the spectral line depends upon the two orbits (energy levels) between which the transition of electron takes place, various spectral lines are obtained. The different wavelengths constitute spectral series which are the characteristic of the atoms emitting them.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
line spectra observed in light emanating from a hydrogen discharge lamp. With Albert Einstein's theory for the photoelectric effect, where a photon has energy proportional to its frequency, Bohr postulated the existence of energy levels in the atom. He assumed

The Energy Level Diagram Of An Element Is Given Blow Identify By Doing Necessary Calculation Which Transition Corresponds To The Emission Of A Spectral Line Of Wavelength 102 7 Nm
Use He discharge lamp for a line with known wavelength (effectively a calibration) Part 2: Balmer series Four visible de-excitation emission lines (i.e. wavelengths) Measure the angle of interference Calculate initial state of transition Tips PHYS 1493/1494/2699: Exp. 7 - Spectrum of the Hydrogen Atom
Using this graphical method we can calculate the wavelength of all three lines of hydrogen to a high degree of accuracy. 2. The spectral lines of hydrogen can also be calculated accurately using a simple relationship. 22 91.15 nm = 11-2n where n can be 3, 4, 5, …, . Calculate for n = 3 through n= 6. 3. Calculate for n = .
energy level to form a line in the Balmer series. i. Calculate the energy of the electron at the energy level n = 4. ii. Determine the wavelength of this transition. Oct 07 21. State the success and failure of Bohr`s atomic model. Calculate the wavelength for a spectral line produced when an electron falls from n = 5 to n = 3.

Calculating Wavelength Of A Spectral Line From An Energy Diagram Practice Chemistry Practice Problems Study Com
Jul 11, 2014 · Wavelength from energy. The formula is. E = hc λ or λ = hc E, where h is Planck's constant. For example, what is the wavelength of a photon that has an energy of. 3.36 × 10⁻¹⁹ J? λ = hc E = 6.626 ×10⁻³⁴J⋅s ×2.998 × 10⁸m⋅s⁻¹ 3.36 × 10⁻¹⁹J = 5.91 × 10⁻⁷ m =. 591 nm.
The electronic energy levels are different for gas-phase atoms and ions of different elements. Therefore, the line spectra produced upon the excitation of a sample can be used to identify the element present. The energies of the photons can be related to the measure wavelength. E = hc/λ = hν E is energy in J c is speed of light = 3.00⋅108 m/s

Expert Verified Calculate The Wavelength In Nanometers Of The Spectral Line Produced When An Brainly Com
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
In quantum mechanics, a shift in the frequency and wavelength of a spectral line implies a shift in the energy level of one or both of the states involved in the transition. The Zeeman effect that occurs for spectral lines resulting from a transition between singlet states is traditionally called the normal effect, while that which occurs when the

The Energy Level Diagram Of An Element Is Given Below Identify By Doing Necessary Calculations Which Transition Corresponds To The Emission Of A Spectral Line Of Wavelength 102 7nm Snapsolve
The diagram below shows the line spectrum of a gas. € 1 Explain how line spectra are produced. In your answer you should describe: •€€€€€€€€how the collisions of charged particles with gas atoms can cause the atoms to emit photons. •€€€€€€€€how spectral lines are explained by the concept of discrete energy ...
(a) Draw the energy level diagram for the line spectra representing Lyman series and Balmer series in the spectrum of hydrogen atom. (b) Using the Rydberg formula for the spectrum of hydrogen atom, calculate the largest and shortest wavelengths of the emission lines of the Balmer series in the spectrum of hydrogen atom. (Use the value of Rydberg constant )
Spectral lines arise as a result of electronic transitions in atoms. The Balmer series is produced by the transitions shown in the following diagram. (a) What transition corresponds to a line A in the spectrum? Explain your answer. 2 (b) Calculate the energy difference, in kJ mol-1, that gives rise to line A, with wavelength 656 nm. 3 SY/91 ...

Calculating Wavelength Of A Spectral Line From An Energy Diagram Practice Chemistry Practice Problems Study Com

The Energy Level Diagram Of An Element Is Given Below Identify By Doing Necessary Calculation Which Transition Corresponds To The Emission Of A Spectral Line Of Wavelength 102 7 Nm Img
The Figure Shows Energy Level Diagram Of Hydrogen Atom I Find Out The Transition Which Results In The Emission Of A Photon Of Wavelength 496 Nm Sarthaks Econnect Largest Online Education Community















0 Response to "43 calculating the wavelength of a spectral line from an energy diagram"
Post a Comment