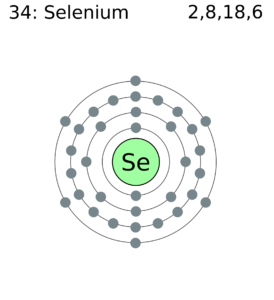
42 selenium electron dot diagram
A step-by-step explanation of how to draw the SeCl2 Lewis Dot Structure.For the SeCl2 structure use the periodic table to find the total number of valence el... Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it.
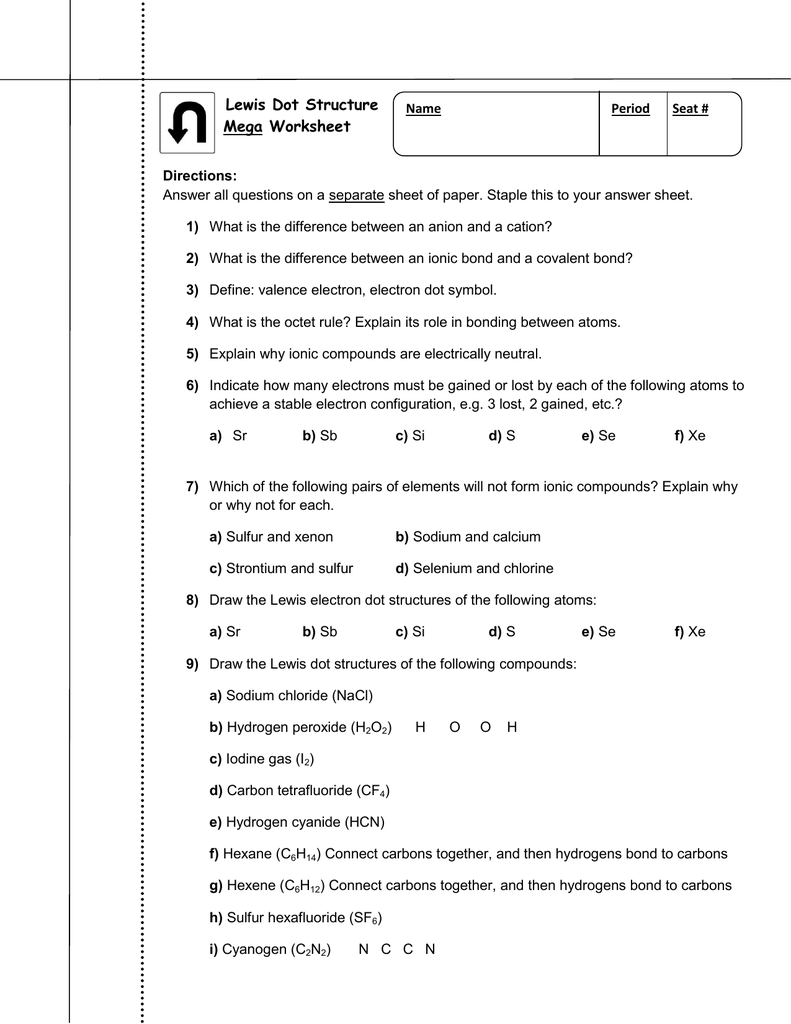
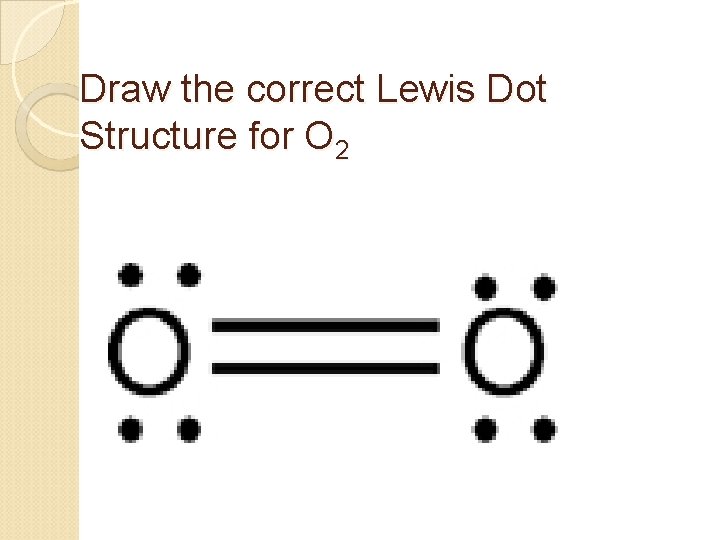
Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are ...
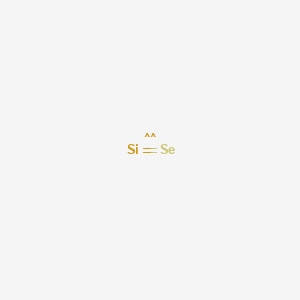
Selenium electron dot diagram
Atomic Structure of Selenium · Atomic Radius: 1.22Å · Atomic Volume: 16.45cm3/mol · Covalent Radius: 1.16Å · Cross Section (Thermal Neutron Capture) σa/barns: 11.7 ... Example 1 · The valence electron configuration for aluminum is 3s 23p 1. So it would have three dots around the symbol for aluminum, two of them paired to ... Example 1: Selenium has 34 electrons. In order to become stable, selenium must obtain the same configuration as xenon. To do this, selenium gains 2 electrons.
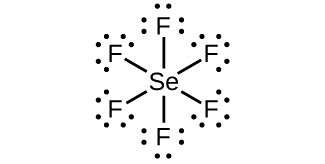
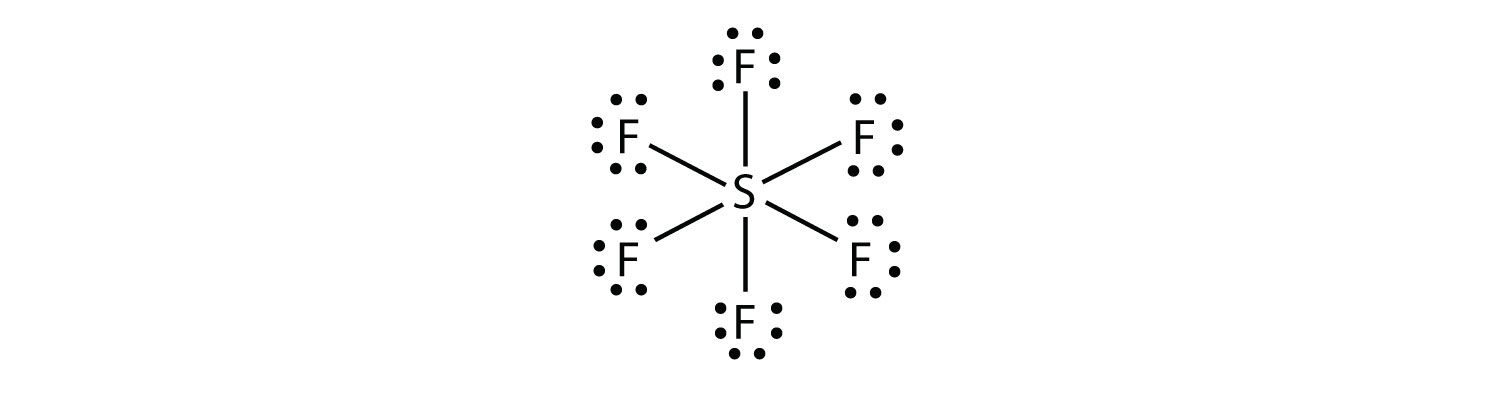
Selenium electron dot diagram. A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the ... Selenium tetrafluoride (SeF4) lewis dot structure, molecular geometry, polar or non-polar, hybridization. Selenium tetrafluoride is an inorganic compound that appears as a colorless liquid having the chemical formula SeF4. It can react with water and forms hydrofluoric acid and selenous acid. Selenium in the SeF4 molecule has a +4 oxidation state. The electron configuration for selenium is 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 4. This configuration is also written as [Ar] 4s 2 3d 10 4p 4, according to Dr. Anne Marie Helmenstine, a contributor to About.com. Example 1. What is the Lewis electron dot diagram for each element? a) aluminum b) selenium . Solution. a) The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: b) The valence electron configuration for selenium is 4s 2 4p 4.
In writing an. Answer to orbital diagram for selenium home / study / science / chemistry / chemistry questions and answers / Orbital Diagram For Selenium. here is the electronic configuration. Z=34 so 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4.Selenium (Se) has an atomic mass of Find out about its chemical and physical properties, states, energy ... Problem. What is the Lewis electron dot diagram for each element? aluminum; selenium; Solution. The valence electron configuration for aluminum is 3s 2 3p 1.So it would have three dots around the symbol for aluminum, two of them paired to represent the 3s electrons: . The valence electron configuration for selenium is 4s 2 4p 4.In the highest-numbered shell, the n = 4 shell, there are six ... Because selenium is in the 16th column on the periodic chart, it has 6 valence electrons. Thus, the Lewis structure (or electron dot structure) for selenium has 6 dots around it. It will look ... Example 1: Selenium has 34 electrons. In order to become stable, selenium must obtain the same configuration as xenon. To do this, selenium gains 2 electrons.
Example 1 · The valence electron configuration for aluminum is 3s 23p 1. So it would have three dots around the symbol for aluminum, two of them paired to ... Atomic Structure of Selenium · Atomic Radius: 1.22Å · Atomic Volume: 16.45cm3/mol · Covalent Radius: 1.16Å · Cross Section (Thermal Neutron Capture) σa/barns: 11.7 ...
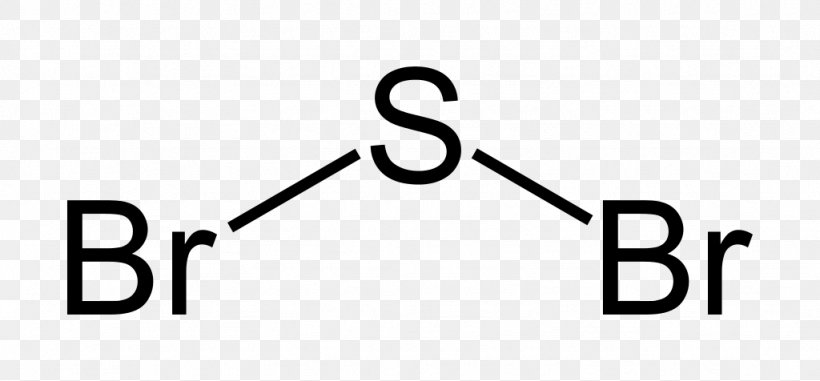
Sulfur Dibromide Lewis Structure Sulfur Dioxide Chemical Compound Png 1024x476px Sulfur Dibromide Area Atom Black And

Electrons In Atoms Electromagnetic Radiation Form Of Energy That Exhibits Both Wavelike Behaviors And Particle Behaviors Ppt Download
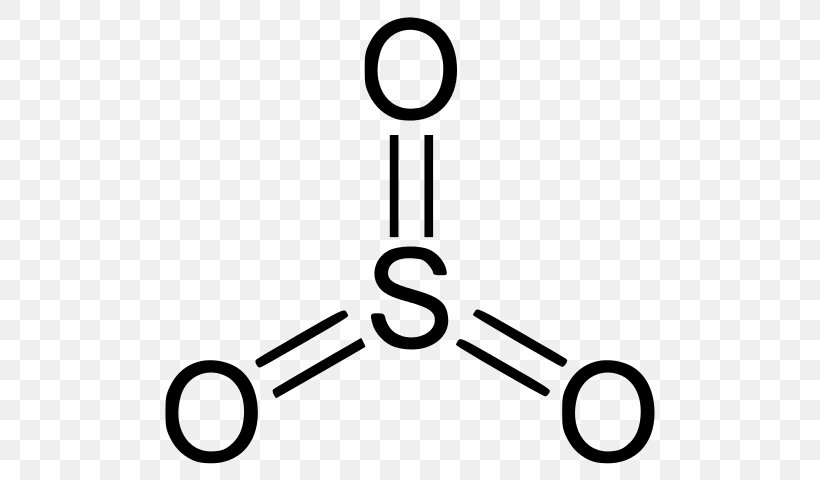
Selenium Trioxide Sulfur Trioxide Lewis Structure Selenium Dioxide Png 524x480px Selenium Trioxide Atom Black And White

Draw The Lewis Structure For The Compound And Include Major Resonance Forms With Formal Changes Sebr 4 Study Com

Lewis Dot Diagrams Name Chem Worksheet 5 7 Jte35633 Worksheets Chemistry 5 7lewisdotdiagrause Your Periodic Table To Answer The Following Questions 1 Lewis Dot Diagrams Name






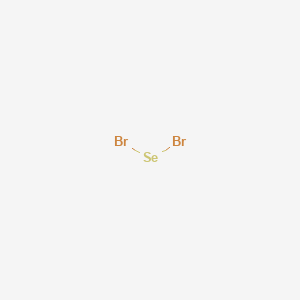














0 Response to "42 selenium electron dot diagram"
Post a Comment