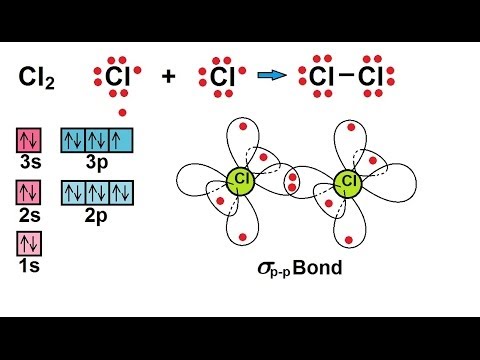
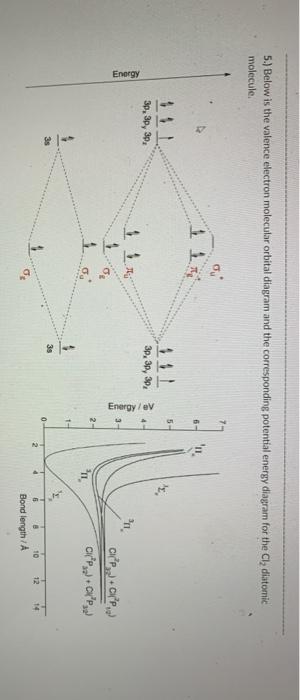
42 cl2 molecular orbital diagram
Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... Chlorine is a commonly used household cleaner and disinfectant. Chlorine is a potent irritant to the eyes, the upper respiratory tract, and lungs. Chronic (long-term) exposure to chlorine gas in workers has resulted in respiratory effects, including eye and throat irritation and airflow obstruction. No information is available on the carcinogenic effects of chlorine in humans from inhalation ...
ClO2 is of C2v point group, so I just read off the C2v character table and got the following group orbitals [where z axis is that of the principal axis, and the outer atoms are aligned such that the y-axis points towards the centre atom]: A1 symmetry, two s orbitals. B1 symmetry, two s orbitals. A1 symmetry, two py orbitals.
Cl2 molecular orbital diagram
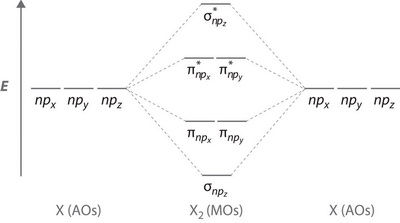
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... Molecular Orbitals for Heteronuclear Diatomic Molecules (MO Theory) When two different atoms are bonded together, their molecule is called heteronuclear molecule. where C1 and C2 are two constants having different values for different atoms. Also the molecular orbitals formed are unsymmetrical due to difference in electronegativities. Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
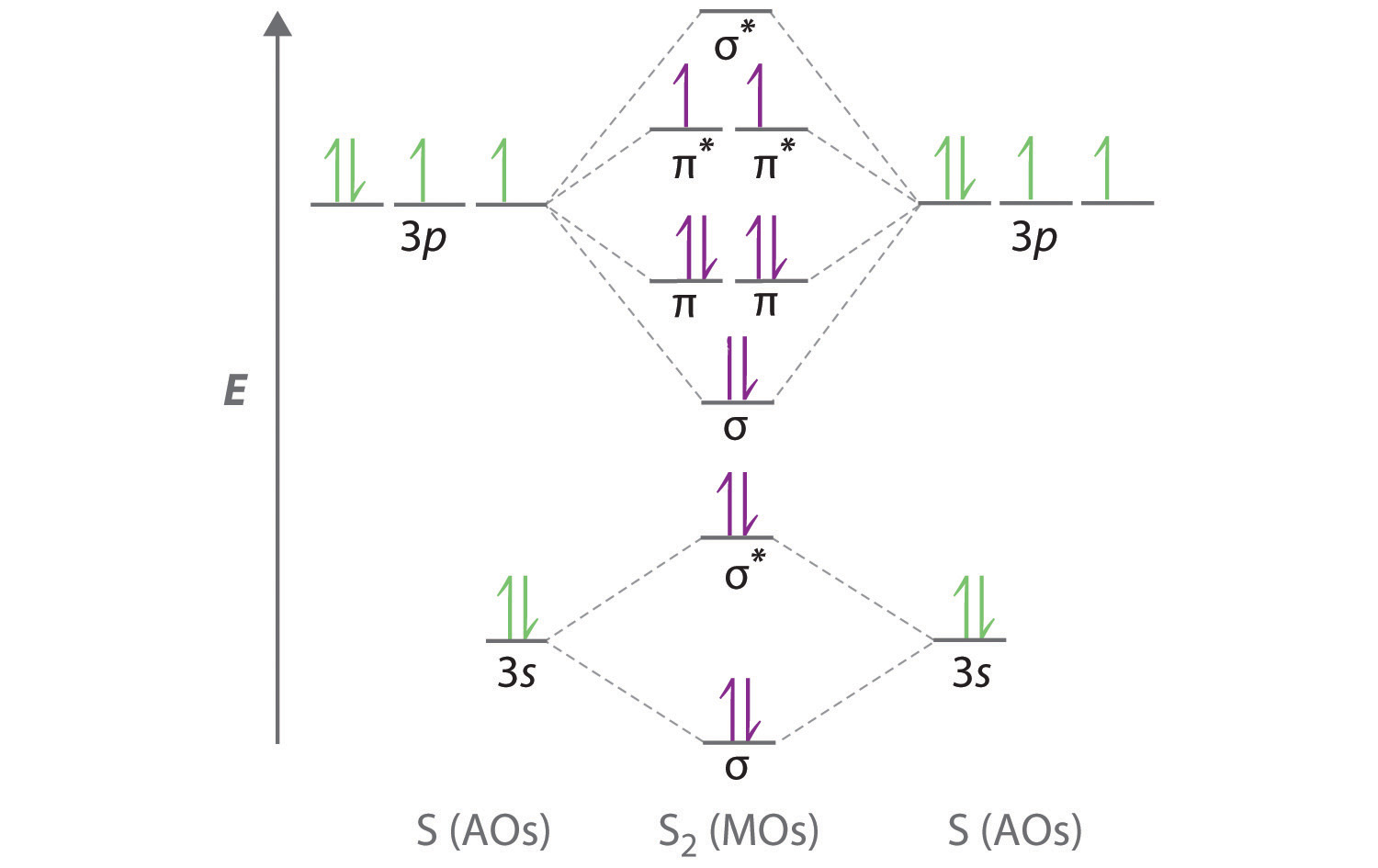
Cl2 molecular orbital diagram. According to Molecular Orbital Theory, its electronic configuration is as follows: $1s2 $*1s2. CH4 + 2 Cl2--> CH2Cl2 + 2HCl Cl-Cl bond energy: 242 kj/mole 1. In picture 2 we show the overlapping p orbitals, which form the bond between the two fl uorine atoms, in red and green gradients. Identify HOMO and LUMO. We're being asked to determine the bond order of Cl2+. For this, we need to do the following steps: Step 1: Calculate the total number of valence electrons present. Step 2: Draw the molecular orbital diagram. Step 3: Calculate the bond order of the molecule/ion. Recall that the formula for bond order is: The molecular orbital diagram for Cl2 ... The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be. Jun 03, 2019 · Molecular Orbital Diagram – Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals’ energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F.
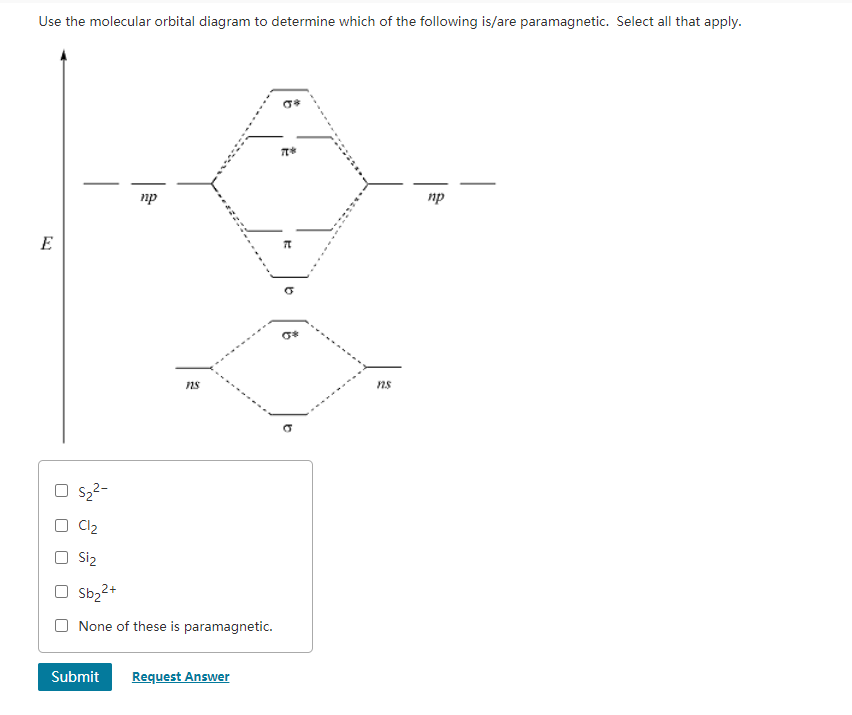
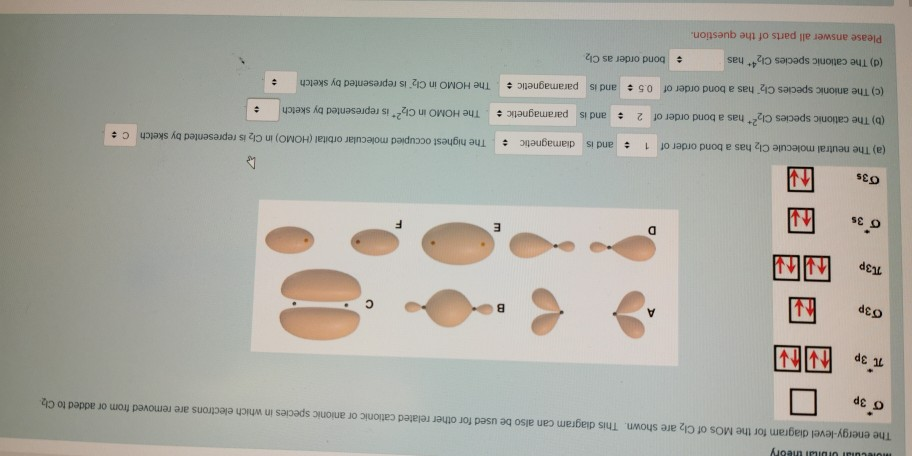
Calculate bond order and describe how the bond distance in this ion would differ from that in Cl2. FREE Expert Solution. 87% (383 ratings) Problem Details. Draw a molecular orbital diagram for Ar 2 +. This ion has been observed in the gas phase. Calculate bond order and describe how the bond distance in this ion would differ from that in Cl 2. Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in Oct 11, 2018 · Cl2 Molecular Orbital Diagram. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . This means that the σ 1 s molecular orbital has a lower energy than either of the hydrogen 1s atomic orbitals. Click here to get an answer to your question how to drew molecular orbital diagram of Cl2. bond order = 1 (like F2) Cl2 has the weakest bond. Download scientific diagram | Molecular orbital diagrams of Cl2, H2O, and Br2. from publication: Theoretical Study of the Potential Energy Surfaces of the Van Der Waals H 2 O−X 2 + (X = Cl or Br ...
Molecular Orbitals An Overview Sciencedirect Topics Draw the atomic orbital diagram for chlorine. Atomic orbital diagram for chlorine. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 kr ar 3d 10 4s 2 4p 6 2. Draw orbital diagrams for the following elements. Chemistry questions and answers. 5) Draw the molecular orbital diagram for Cl2. Assume 3s and 3p orbitals show similar LCAO as 2s and 2p orbitals (Label increasing energy using an arrow next to the diagram. Label each atomic and each molecular orbital) Calculate the bond order and give the molecular orbital configuration for the valence electrons. An electron from the 22 orbital and three other electrons from 2p orbitals participate in forming bonds. Thus the hybridization of Carbon atom in CH2Cl2 is sp3. Molecular Geometry of Dichloromethane. It is comparatively easy to understand the molecular geometry of a compound after knowing its Lewis structure and hybridization. Mar 11, 2018 · Cl2 molecular orbital diagram. In contrast to crystal field theory, molecular orbital included the covalent nature of the metal-ligand bond interaction. Energy of. Cl atom has 17 electrons, so chlorine molecule has (Cl2) has 34 electrons. so, bond order of chlorine molecule is 1.
Draw the molecular orbital diagram for n2 ion and calculate the bond order. Here is the full molecular orbital diagram for n2. The 2px orbitals on each atom combine to make a pi bonding and a pi antibonding molecular orbital in the xz plane. They werent drawn that way on this diagram but they should be.
COCl2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity. COCl2 is a chemical compound, known by the name 'phosgene'. Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of 98.92 gram/mol. It is non-flammable in nature and bears a suffocating odor.
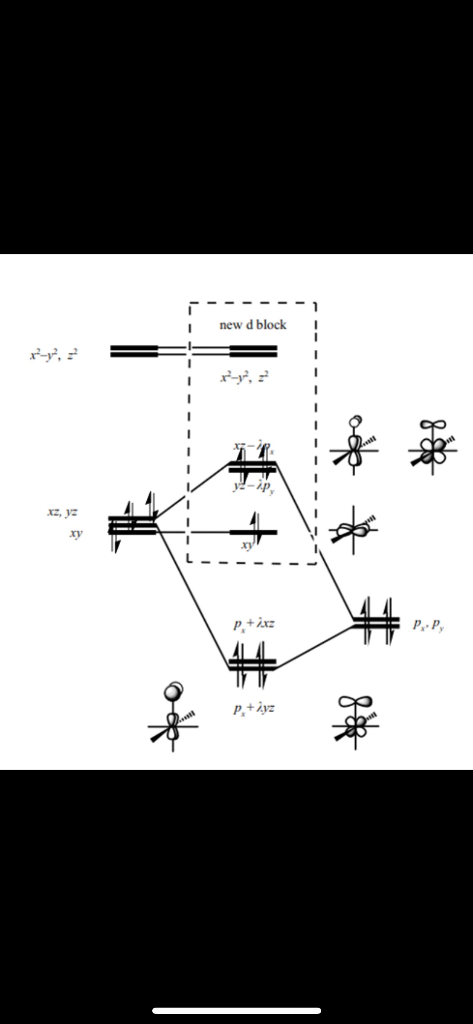
d orbitals •l = 2, so there are 2l + 1 = 5 d-orbitals per shell, enough room for 10 electrons. •This is why there are 10 elements in each row of the d-block. σ‐MOs for Octahedral Complexes 1. Point group Oh 2. The six ligands can interact with the metal in a sigma or pi fashion.
Answer (1 of 2): B.O. of Cl2 = 1
Molecular orbital diagram explains the chemical bonding in the molecules in terms of molecular orbital theory. In a molecular orbital diagram, the diagram of molecular orbital energy levels is shown as horizontal lines. Degenerate orbitals (orbitals having the same energy) are shown side by side in these diagrams.
Answer (1 of 2): B.O. of Cl2 = 1
Molecular geometry of Cl2 Hybridization of Cl2. The hybridization of each chlorine atom in the Cl2 lewis structure is Sp³. It means it has one s orbital and three p orbital. And this makes four hybrid orbitals. In order to find the hybridization of the chlorine atom in the Cl2 molecule, we have to find its steric number.
Question: Draw the molecular orbital diagram for Cl2. Assume 3s and 3p orbitals show similar LCAO as 2s and 2p orbitals (Label increasing energy using an arrow next to the diagram. Label each atomic and each molecular orbital) Calculate the bond order and give the molecular orbital configuration for the valence electrons.
This video creates the full molecular orbital diagram for dichlorine, shows the coordinate system, the point group, the symmetries of the atomic orbitals, th...
2 Cl2 + 2 NaHCO3 ——> Cl2O + 2CO2 + 2NaCl + H2O 2 Cl2 + Na2CO3 ——-> Cl2O + CO2 + 2 NaCl ... With this information, molecular geometry, hybridization, and molecular orbital diagram can be studied further. To begin studying the Lewis structure of dichlorine monoxide, it is first crucial to study the Lewis structures of the participating ...
Fluorine Molecular Orbital Diagram – Cl2, Br2, I2. A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in . bond order = 1 (like F2) Cl2 has the weakest bond.
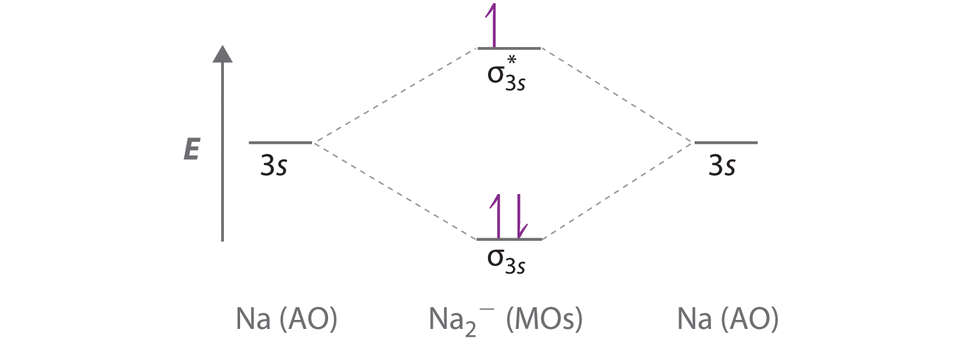
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules An atomic orbital is located on a single atom. When two (or more) atomic orbitals overlap to make a bond we can change our perspective to include all of the bonded atoms and their overlapping orbitals. Since more than one atom is involved, we refer to these orbitals as molecular orbitals.
Molecular Orbital Diagrams simplified. Megan Lim. Oct 26, 2016 · 3 min read. Drawing molecular orbital diagrams is one of the trickier concepts in chemistry. The first major step is understanding ...
Molecular Orbitals for Heteronuclear Diatomic Molecules (MO Theory) When two different atoms are bonded together, their molecule is called heteronuclear molecule. where C1 and C2 are two constants having different values for different atoms. Also the molecular orbitals formed are unsymmetrical due to difference in electronegativities.
Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...

2 A Molecular Orbital Diagram For A Tetrahedral Transition Metal Complex Is Shown Below Show How Homeworklib
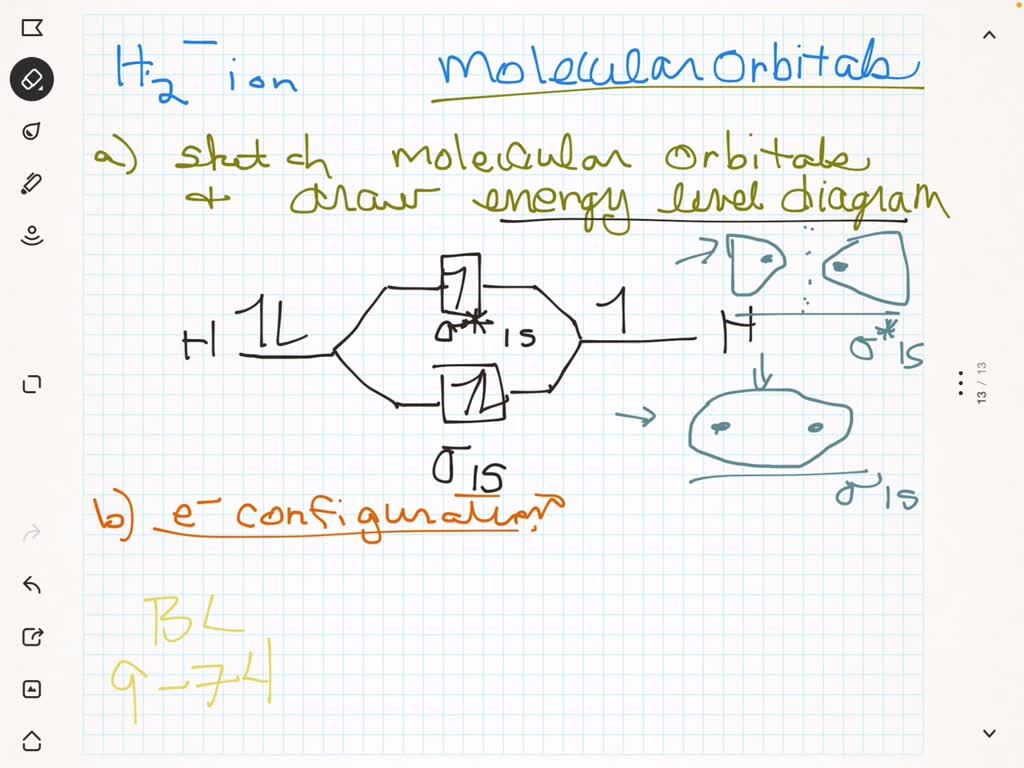
Solved A Sketch The Molecular Orbitals Of The Mathrm H 2 Ion And Draw Its Energy Level Diagram B Write The Electron Configuration Of The Ion In Terms Of Its Mos C Calculate The Bond Order

Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

Pdf Two Dimensional H2o Cl2 And H2o Br2 Potential Surfaces An Ab Initio Study Of Ground And Valence Excited Electronic States Semantic Scholar









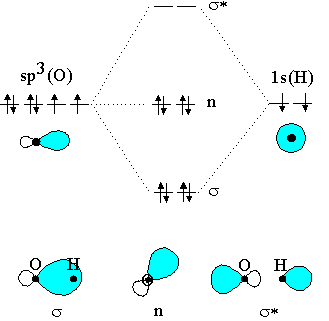








0 Response to "42 cl2 molecular orbital diagram"
Post a Comment