41 h2+ molecular orbital diagram
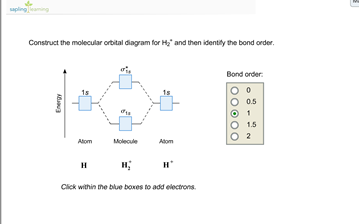
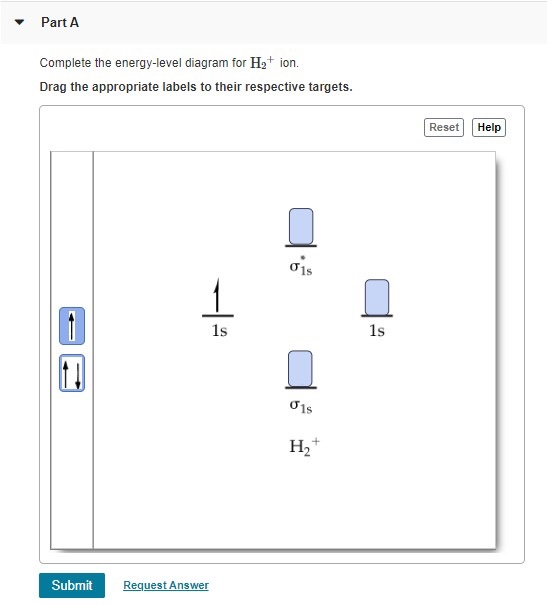
Chemistry questions and answers. Construct the molecular orbital diagram for H2+ If all of the orbitals are unoccupied, place the corresponding token in the bin underneath the answer bank. os Answer Bank 11 1 1s 1s Energy 015 all of the orbitals are unoccupied Atom Molecule Atom HT h H Identify the bond order. 0 0.5 1 1.5 2. Mo · Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order. chemical bonding molecular orbitals of h2 and he2 as before the greater the number of these nodal planes the more the electrons that occupy the orbitals are excluded from the region between the nuclei and hence the higher the energy the resulting molecular ...
by S Nagaoka · 2012 · Cited by 2 — and HF. Some contour representations and molecular orbital energy-level diagrams thus obtained are not consistent with the.

H2+ molecular orbital diagram
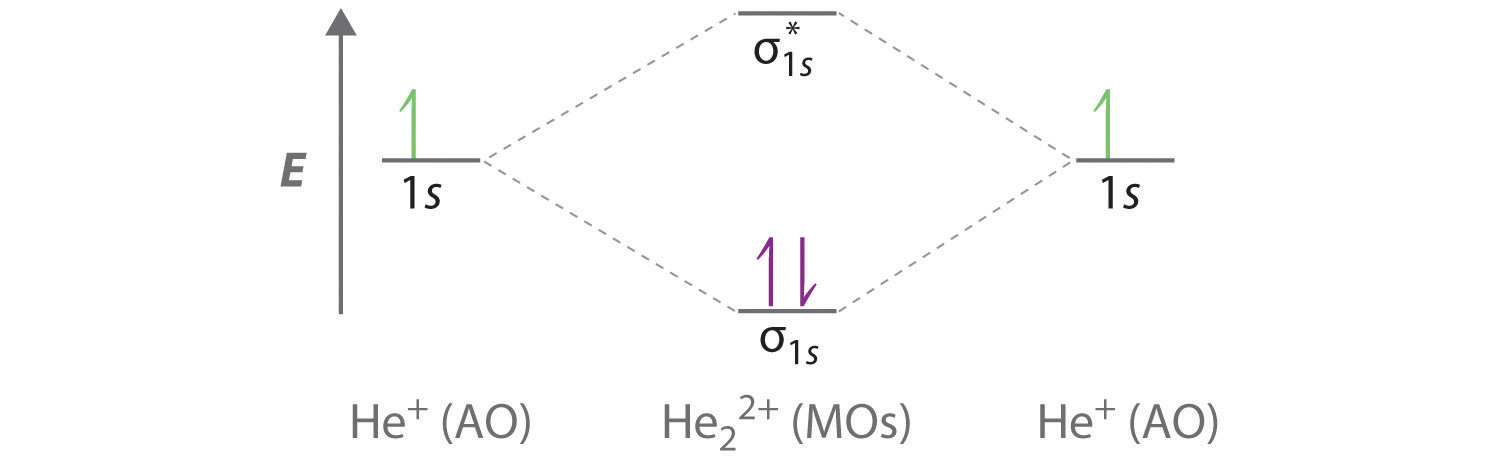
Solution for Consider the H2+ ion. (a) Sketch the molecular orbitals ofthe ion and draw its energy-level diagram. (b) How manyelectrons are there in the H2+… Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+. Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic ... Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can “mix” (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ ...
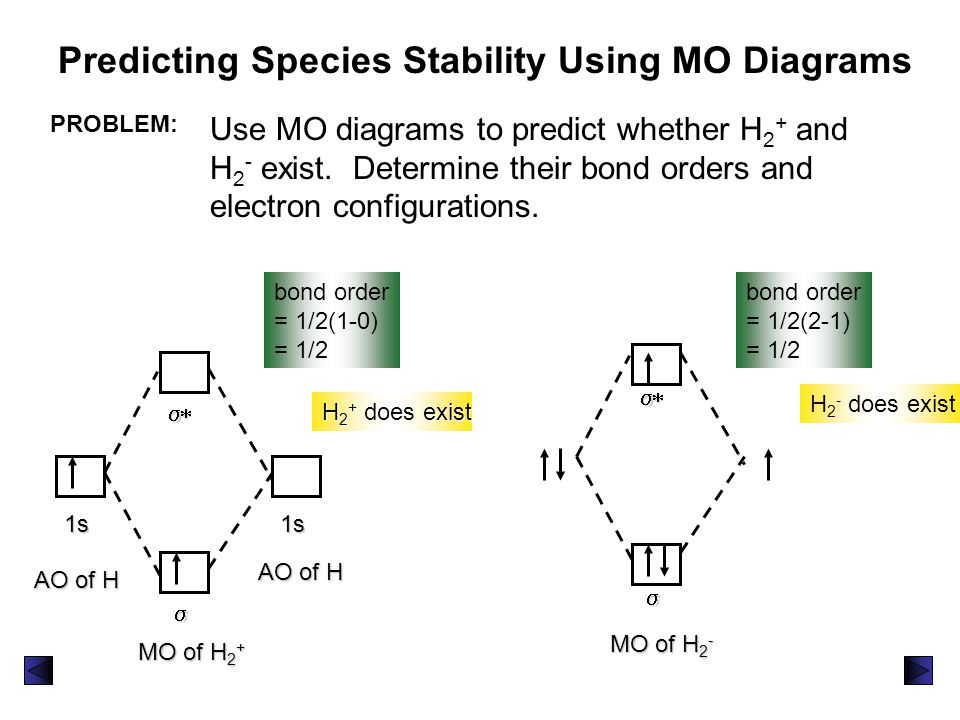
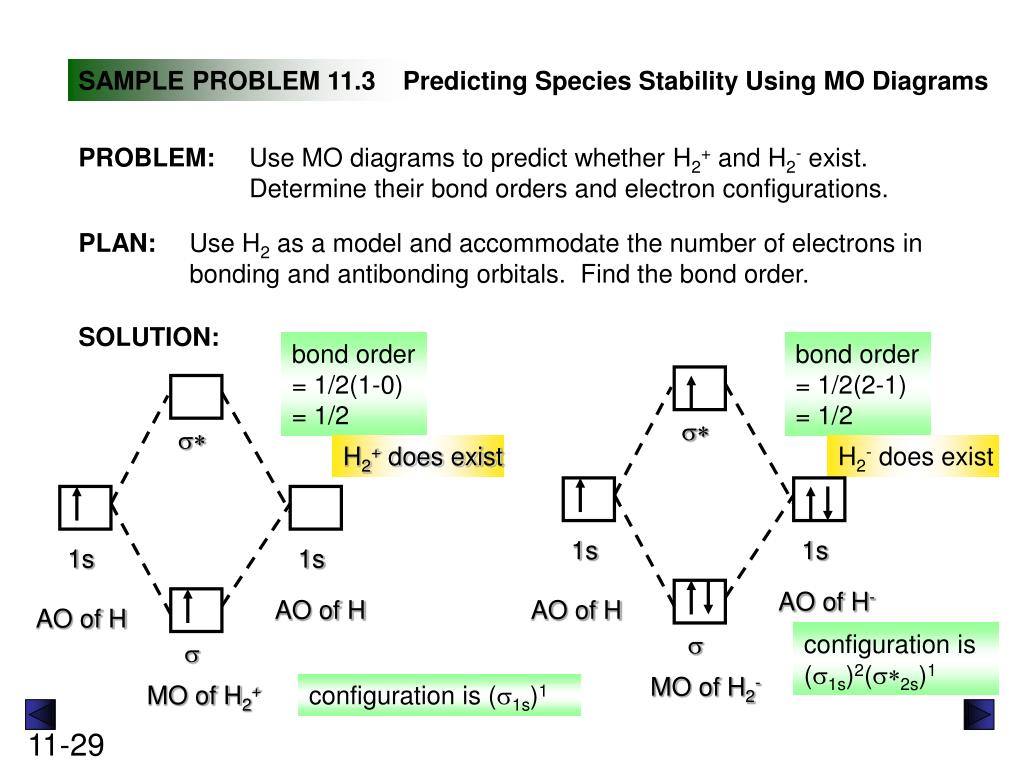
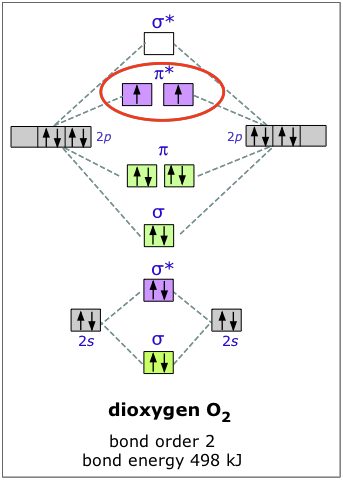
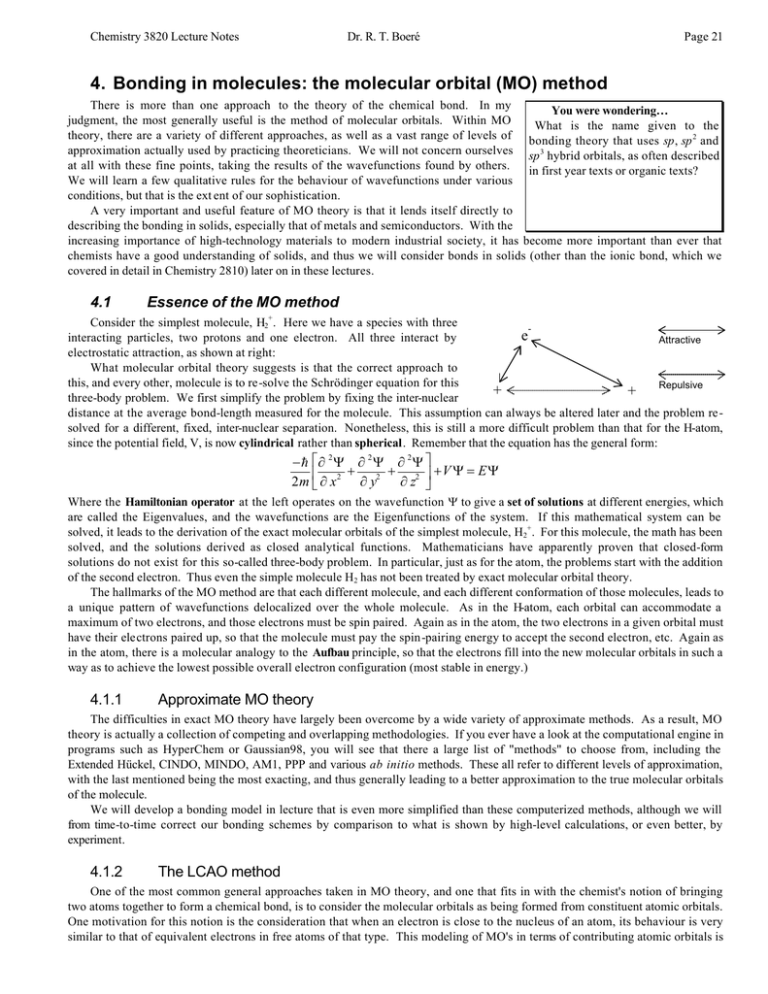
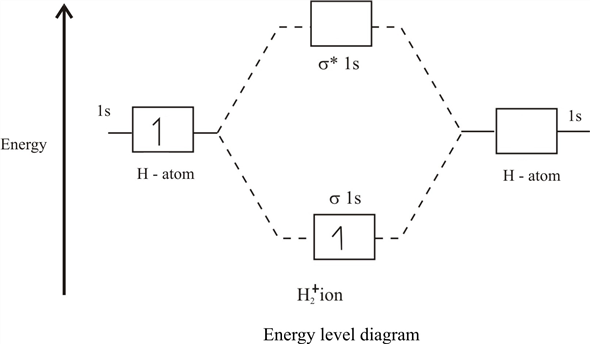
H2+ molecular orbital diagram. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ... H2 molecular orbital diagram. Molecular orbitals of h 2 and he2. Many theories have been developed using them as models. This results in a bonding sigma mo σ 1s. Bonding and anti bonding molecular orbitals in h 2. Dashed lines connect the parent atomic orbitals with the daughter molecular orbitals. Get access to the latest Bonding in Homonuclear Diatomic Molecules: H2, H2+, H2-, He2, He2+ prepared with IIT JEE course curated by Megha Khandelwal on Unacademy to prepare for the toughest competitive exam. The energy curves for ψ + and ψ-reveal the following properties of the ion H 2 +. The curve for ψ + refers to the ground state of the molecule where a minimum energy is found for a nuclear distance of approximately 2a o (i.e. 100pm). Thus, H 2 + should exist as a stable molecule. The calculated bonding energy is 1.77 eV. This is a quite satisfying result; from experiments we get 2.77 eV.
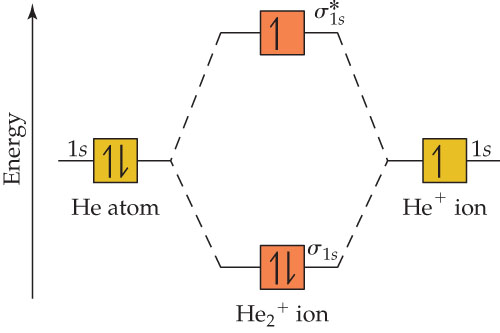
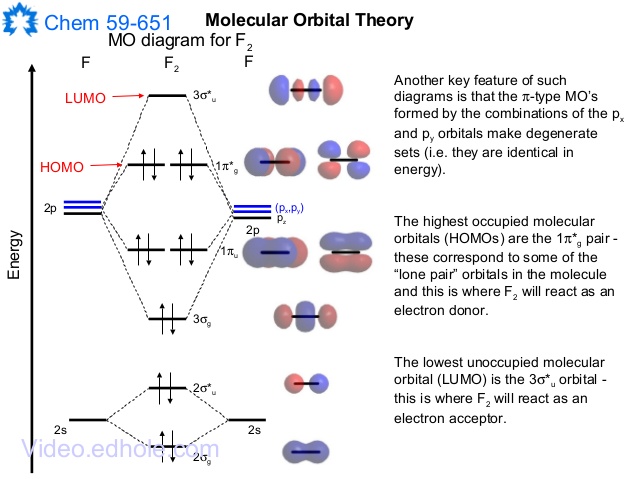
brings only a 1s orbital to the mixing. Just as in the molecules H2 and H2 +, the molecular orbitals for He2 are created by the combination of two 1s atomic orbitals (Fig. 1.46). Each helium atom brings two electrons to the molecule, though, so the electronic occupancy of the orbitals will be different from that for H2 or H2 +. The Answer to Construct the molecular orbital diagram for H2+ and then identify the bond order.... A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ... May 18, 2018 - Brainly.in is a part of the largest social network for studying in a group. We provide the best tools for mutual help with school subjects. Join us!
August 15, 2020 - Check out our new LibreCommons search portal · Describe the hydrogen molecule in light of the following: FREE Answer to Construct the molecular orbital diagram for H2+ and then identify the bond order.1 answer · 0 votes: Molecular orbitals: Molecular orbitals are formed by linear combination of atomic orbitals. Atomic orbitals and molecular orbitals of a molecule can be ... 14+ H2 Molecular Orbital Diagram. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. The filled molecular orbital diagram shows the number of electrons in both bonding and antibonding molecular orbitals. Principle 2 & 3: This interaction ... to the molecular orbitals, may also be represented in the form of an orbital (electron) energy diagram which shows the relative energies of the orbitals. In the specific case of hydrogen each of the isolated atoms has one electron in its 1s orbital and when the atoms combine to form H2 the two electrons ...
0:15 Molecular Orbital Diagram of Hydrogen Molecule1:39 Molecular Orbital Diagram of Helium Molecule2:54 Molecular Orbital Diagram of Lithium Molecule4:00 Mo...

Using Lcao Method For The Formation Of Molecular Orbitals In Case Of Homonuclear Diatomic Hydrogen Molecule From Chemistry Chemical Bonding And Molecular Structure Class 11 Haryana Board English Medium
Simple Molecular Orbitals - Sigma and Pi Bonds in Molecules bond order = (number of bonding electrons) - (number of antibonding elect rons) 2 = amount of bonding 1sa hydrogen molecule = H2 LUMO HOMO σ = 1sa + 1sb = bonding MO = potential energy higher, less stable lower, more stable LUMO = lowest unoccupied molecular orbital HOMO = highest ...
Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Molecular orbital diagram h2. Because of their simplicity they have been extensively studied. Two superpositions of these two orbitals can be formed one by summing the orbitals and the other by taking their difference. Construct the molecular orbital diagram for h2 and then identify the bond order.
March 10, 2016 - Molecular orbital energy level diagrams of certain diatomic Homo nuclear molecules and molecular ions · The filling of molecular orbitals is governed by the following principles. ... (iii)Hund's rule of maximum multiplicity. Now, let us consider some examples of homo nuclear diatomic molecules. 1. Hydrogen molecule, H2...

Draw The Molecular Orbital Diagram Of H2 02 And N2 Molecules And Calculatetheir Bond Orders As Brainly In
Answer to Construct the molecular orbital diagram for H2- and then identify the bond order. Bond order: Click thin the blue boxes. The hydrogen atom is the simplest atom, and its molecule \ (\ce {H2}\) is get a sigma (s) bonding orbital, denoted as s1s in the diagram here. Chemical bonding - Molecular orbitals of H2 and He2: The procedure can ...

Construct The Molecular Orbital Diagram For He2 And Then Identify The Bond Order Bond Order Click Homeworklib
Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Each boron atom has one 2s and three 2p valence orbitals. In order to predict the bond order molecular orbital diagram for h2 is to be drawn. Molecular orbitals of h 2 the molecular orbital approach is one explanation for the ceh h bond.
When An Electron Of H2 Is Promoted To The Excited State Does The Molecule Continue To Exist Or Does Its Bond Break Quora
Professor Patricia Shapley, University of Illinois, 2011
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.The Hydrogen Molecule Ion H2+Molecular Orbital Diagrams of Diatomic Molecules - Chem
March 2, 2021 - https://chem.libretexts.org/@a...A_molecules/2.3%3A_Homonuclear_Diatomic_Molecules_-_Molecular_Orbital_(MO)_Theory/2.3b%3A_MO_theory_of_bonding_in_H ... Note that there is a nodal plane in the anti-bonding MO. ... For H2, bond order = 1/2 (2-0) = 1, which means H2has only ...
The next molecule in the series HF, H2O and H3N, is H4C (methane) - which was discussed earlier - and unlike the other three molecules has no non-bonding orbitals. ... bonds in close proximity will often interact. Some of the delocalized molecular orbitals that result will be stabilized, while ...
This problem has been solved! A.Construct the molecular orbital diagram for H2^2+ and then identify the bond order. B.Construct the molecular orbital diagram for H2 and then identify the bond order. C.Construct the molecular orbital diagram for H2^- and then identify the bond order. D.Construct the molecular orbital diagram for H2^+ and then ...
A molecular orbital explicitly describes the spatial distribution of a single electron orbitals, and σ∗. 1s is higher in energy. Draw this out using an energy level diagram: 2 He2 has bond order 0 [ (2 − 2)/2 = 0], and we can make H+. 2,. H−.A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical ...
Molecular orbitals of H. The simplest neutral molecule is molecular hydrogen, H 2, which consists of two electrons and two protons. The molecular orbital Hamiltonian in this case is the same as it is for the molecular hydrogen ion and the molecular orbitals are the same as for the molecular ion. HH2 mo =− ℏ2 2me ∇2− e2 4πϵ0|.
Well, build the molecular orbital (MO) diagram. Each hydrogen atom contributes one electron, and thus, "H"_2^(-) has three electrons while "H"_2^(+) has one. Each hydrogen atom contributes one 1s atomic orbital, and thus, the orbitals overlap according to MO theory to form one sigma_(1s) and one sigma_(1s)^"*" MO by conservation of orbitals.
Optimizing H2+ Molecular Orbital, H2, and Configuration Interaction ... which is a molecular orbital diagram which some of you may already have used without ...
November 23, 2015 - 12/20: Andy's paper on supported nanocrystal catalysts is published in Chem. Mater.! 10/20: Our joint paper with the Klimov group using ALD to produce CMOS circuit elements from CIS quantum dot films is published in Nature Comms. 9/20: Our paper with Adam Moule's group on electron tomography ...

Use Molecular Orbital Theory To Determine Whether He2 2 Or He2 Is More Stable Draw The Molecular Orbital Diagram For Each And Explain Study Com
chemical bonding - chemical bonding - Molecular orbitals of H2 and He2: The procedure can be introduced by considering the H2 molecule. Its molecular orbitals are constructed from the valence-shell orbitals of each hydrogen atom, which are the 1s orbitals of the atoms. Two superpositions of these two orbitals can be formed, one by summing the orbitals and the other by taking their difference.
The molecular orbital energy-level diagram, which is a diagram that shows the relative energies of molecular orbitals, for the H2 molecule is shown in Figure A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear ...
Answer (1 of 2): In order to predict the bond order, molecular orbital diagram for H2- is to be drawn. According to MOT number of atomic orbitals combined is equal to total number of molecular orbitals formed.Electronic configuration of H is 1s1. when two hydrogen atoms come closer, then on combi...
November 17, 2019 - Solution for Construct the molecular orbital diagram for H2. Identify the bond order. 0 0.5 1 1.5 2
Construct the molecular orbital diagram for h2 and then identify the bond order. Construct the molecular orbital diagram for h2. The procedure can be introduced by considering the h2 molecule. Description of the molecular orbitals of the h2 molecule with an introduction to molecular orbital diagrams. Click within the blue boxes to add electrons.
Hydrogen Molecular Orbital Diagram Atomic hydrogen has 1 electron in a 1s orbital. Of course, there are 2s, 2p, 3s, 3p, etc. empty orbitals at higher energy. Let's just consider the 1s orbitals. Remember that an orbital is a mathematical function that describes the probability of finding an electron in space.
H2 molecular orbital diagram. Qualitative mo theory orbital diagram for homonuclear diatomics. The bond order of a diatomic molecule is defined as one half the difference between the number of electrons in bonding orbitals nb and the number of electrons in antibonding orbitals na. A double covalent bond a bond order of two.

Construct The Molecular Orbital Diagram For H2 And Then Identify The Bond Order Bond Order Click Homeworklib
Molecular orbital theory considers electrons to be distributed over the entire molecule, while valence bond theory considers electrons to be localized to a bond. The image provided shows two 3px orbitals. Predict what type of molecular orbital will result. σ3p. If an electron became excited, it could. jump from the sigma (σs) or bonding ...
In order to continue enjoying our site, we ask that you confirm your identity as a human. Thank you very much for your cooperation
UCI Chem 131A Quantum Principles (Winter 2014) Instructor: A.J. Shaka, Ph.D Description: This course provides an introduction to quantum mechanics and principles of quantum chemistry with applications to nuclear motions and the electronic structure of the hydrogen atom.
Molecular Orbital Diagram for Hydrogen Gas (H2).Fill from the bottom up, with 2 electrons total.Bonding Order is 1, and it is Diamagnetic.sigma2s(2)Check me ...
8:50... is the bond order of H2 , H2+ and H2- Molecular species. we will also discuss molecular orbital diagram for ...1 Jul 2021 · Uploaded by My chemistry teacher
Molecular orbital diagram of h2. When creating the molecular orbitals from the p orbitals notice the three atomic orbitals split into three molecular orbitals a singly degenerate σ and a doubly degenerate π orbital. Molecular orbitals of h2 and he2. Bonding mos antibonding mos and bond order. Evaluate the ground state electronic energy based ...
Molecular orbitals of h2 and he2. The molecular orbital energy level diagram for the h2 ion. Suppose that the ion is excited by light so that an electron moves from a lower energy to a higher energy molecular orbital. πε and jr k r mr lr defined explicitly in atkins. Sketch the molecular orbitals of the h2 ion and draw its energy level diagram.
The resulting electron configuration ... (H2): 1σg2. Sometimes, the letter n designates a non-bonding orbital. The presence of a filled antibonding orbital, after fulfilling the conditions above, indicates that the bond in this case does not exist. The bonding diagram for the ...
Molecular Orbital (MO) Theory of the H2 molecule: Following the MO treatment of H2+, assume the (normalized) ground electronic ... Spin‐orbitals of type 1 and 3 have the same symmetry, and therefore can “mix” (to give improved wavefunctions and energy eigenvalues): 1 ψψ αβ ...
Answer to Construct the molecular orbital diagram for He2 and then identify the bond order. Bond order: Click within the blue boxe. A molecular orbital explicitly describes the spatial distribution of a single Energy Level Diagrams He2 has bond order 0 [(2 − 2)/2 = 0], and we can make H+.
Solution for Consider the H2+ ion. (a) Sketch the molecular orbitals ofthe ion and draw its energy-level diagram. (b) How manyelectrons are there in the H2+…
Solved Write Out The Molecular Orbital Theory Electronic Configuration Of H22 And The Bond Order Course Hero











0 Response to "41 h2+ molecular orbital diagram"
Post a Comment