41 electron dot diagram for nitrogen
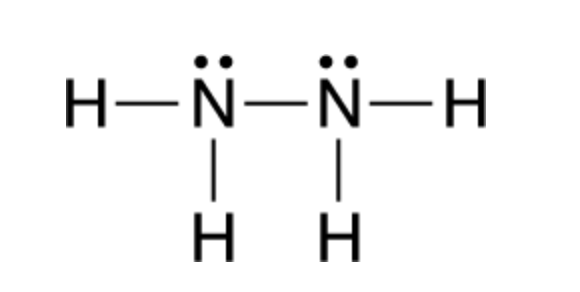
1. Draw an "electron dot" diagram showing the first 18 elements in the periodic table. 2. Explain how the electron dot diagram is similar for families in the periodic table. 3. Draw an electron dot diagram showing the formation of ions and ionic compounds. 4. Explain how hydrogen can be considered as behaving like a metal or a nonmetal. Nitrogen trichloride (NCl3) lewis structure contains three N-Cl bonds. There is one lone pair on nitrogen atom and three lone pairs on each chlorine atom. Lewis structure of NCl3 can be drawn by using valence electrons of nitrogen and chlorine atoms. Also, there are no charges on atoms in NCl3. Steps of drawing the lewis structure of NCl3 are explained in detail in this tutorial.
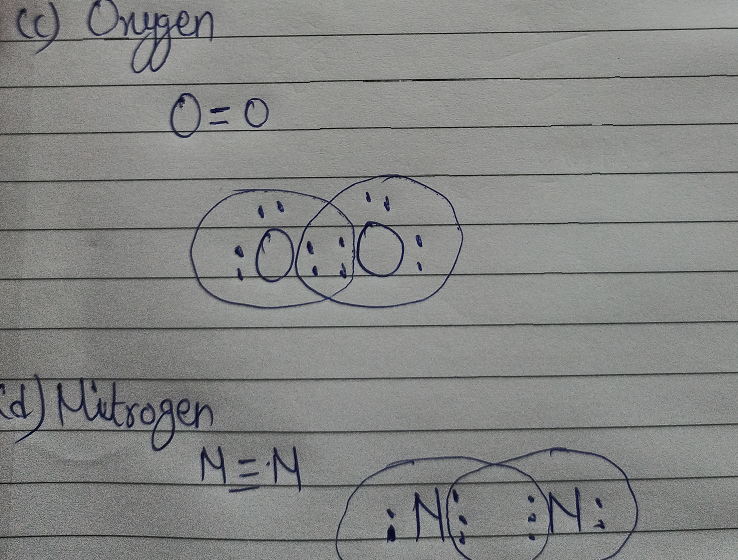
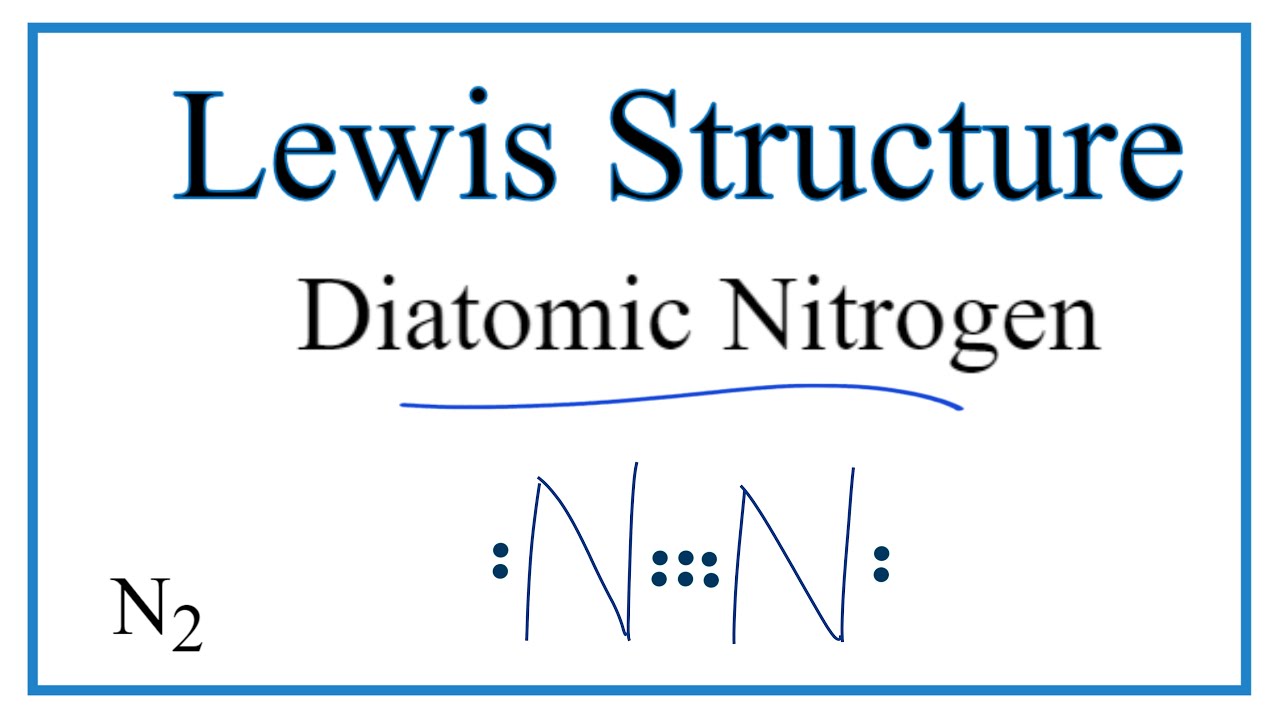
on Lewis Dot Diagram For N2. Plucked this image from google. There are 3 dots (electrons) in the middle for each Nitrogen atom because Nitrogen molecules form triple. The Lewis Structure for N2 looks easy at first. The problem is that there aren't enough valence electons to give both Nitrogen atoms an octet. You'll need to use .

Electron dot diagram for nitrogen
A step-by-step explanation of how to draw the NI3 Lewis Dot Structure (Nitrogen Triiodide).For the NI3 structure use the periodic table to find the total num... Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom. Draw the electron dot structure of Nitrogen molecule [N = 7] Medium. Open in App. Solution. Verified by Toppr. Nitrogen molecule N = 7 ...
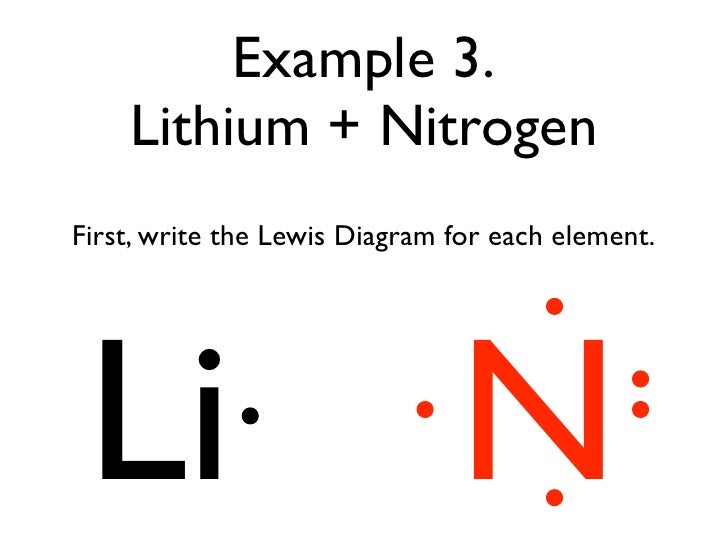
Electron dot diagram for nitrogen. nitrogen electron dot diagram. aluminum electron dot diagram. silicon electron dot diagram. phosphorus electron dot diagram. sulfur electron dot diagram. calcium electron dot diagram. argon electron dot diagram. full outer shell. 8 electrons (except for Helium, which is 2); Subjects. Arts and Humanities. Languages. Math. Science. The Lewis structure of nitrogen dioxide is also interesting because there is a single unpaired valence electron on the central nitrogen atom. Compounds with unpaired electrons are sometimes referred to as "free radicals." This unpaired electron explains nitrogen dioxide's reactive behavior as it has a strong desire to fill this open ... Electron Dot Diagrams Chemistry for Non. Describe the electron dot diagram system of representing structure. Recall that the valence electrons of an atom are the electrons located in the highest occupied principal energy level. nitrogen, 1 s 2 2 s 2 2 p 3, 5 valence electrons. N2O4 Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and Polarity. Dinitrogen tetroxide (N2O4) is commonly known as nitrogen tetroxide (NTO). It is colorless in solid form while in liquid and gaseous form; it has a characteristic reddish-brown color. It has an unpleasant, irritating acid-like smell.
Transcript: For the HCN Lewis structure we have one valence electron for Hydrogen, we have four for Carbon, and we have five for Nitrogen, for a total of ten valence electrons for the HCN Lewis structure. We'll put the Carbon in the center, because it's less electronegative than the Nitrogen, and Hydrogens always go on the outside of Lewis structures. What is the electron dot diagram for nitrogen? Note: Nitrogen is in Group 5 (sometimes called Group V or Group 15). Since it is in Group 5 it will have 5 valence electrons. When you draw the Lewis structure for Nitrogen you'll put five "dots" or valance electrons around the element symbol (N). This is the structure of ammonia or \[N{{H}_{3}}\].-In ammonium ion, the lone pair on nitrogen atoms of ammonia has the ability to fully share its pair with hydrogen ions, thus forming a coordination bond with nitrogen. Nitrogen will attain a positive charge on it. Thus it becomes an ammonium ion. The electron dot diagram is shown below. N2 Lewis Structure The N2 Lewis structure has a triple bond between two nitrogen atoms. According to the octet rule, nitrogen atoms need to bond three times. The N2 molecule is diatomic, meaning that two atoms of the same element are connected in a pair. N2 Lewis Structure Setup It's easiest to think in terms of […]
Electron dot diagram of a Nitrogen atom. Electron dot diagram also called lewis structure which represents the valence electrons of atoms. As, from the Bohr diagram of Nitrogen, we got to know, it has 5 valence electrons. So, just represent these 5 valence electrons around the Nitrogen atom as a dot. Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha. Steps to Draw the Lewis structure of N2. Below is the electron dot structure for a Nitrogen molecule: In the Periodic Table, Nitrogen is placed in Group 5 across Period 2. Thus, as per the electronic configuration of the element i.e. 2,5, it has five electrons in its outermost valence shell. As per the molecule N2, it has two atoms of Nitrogen. How to Draw the Lewis Structure of N2 - with explanation!Check me out: http://www.chemistnate.com
The Lewis structure of Nitrogen atom can be drawn if one knows the number of valence electrons of Nitrogen. The electronic configuration of Nitrogen is 1s^2 2s^2 2p^3 The nitrogen atom has five electron present in 2s and 2p subshell and these electrons are called valence electrons. Nitrogen atom has 5 valence electrons, so its Lewis dot symbol for N is This video shows how to use the periodic ...
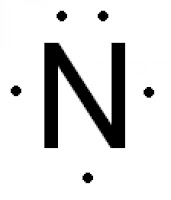
The Lewis dot structure of a nitrogen atom would be the capitol letter N with the five valence electrons represented by two dots above it, one to the left right and bottom of it. .. . N . .
Electron Dot Diagram For Nitrogen. It is the lightest pnictogen and at room temperature it is a transparent odorless diatomic gas. Nitrogen dioxide does not have a single lewis structure on account of its relatively strange electron configuration. Nitrogen Gas Lewis Dot Structure For Nitrogen Gas.
The total valence electron available for the NI3 lewis dot structure is 26. The hybridization of NI3 is Sp³. Nitrogen triiodide is slightly polar in nature. The molecular geometry of NI3 is trigonal pyramidal and its electron geometry is tetrahedral. Lewis structure of NI3 contains 1 lone pair and 3 bonded pairs.
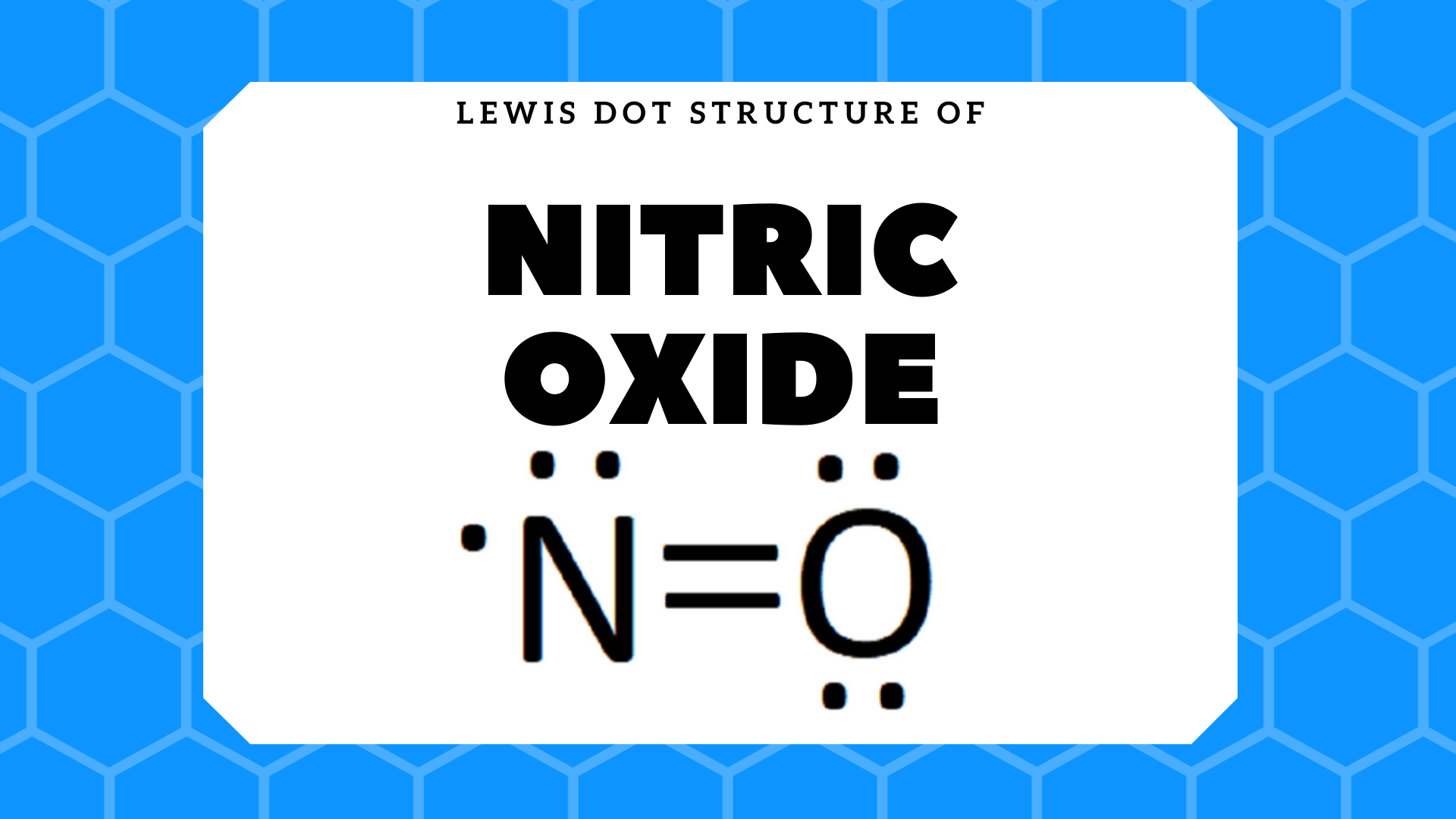
what is the Lewis structure of NO nitrogen monoxide, Lewis structures of nitrogen monoxide, Lewis electron dot structures of nitrogen monoxide, electron dot structures of nitrogen monoxide, NO Lewis structures, NO electron dot structures, NO dot structures, pi an d, for the draw, lewis no, dot structure of NO, electron dot lewis structure of NO, resonance structures of NO, ap chemistry lewis ...
www.toppr.com
Answer (1 of 2): NO3- is the nitrate ion, and the conjugate base of nitric acid.Two of the three oxygens bound to the nitrogen atom have their three outer lone pairs, with a sigma bond to the nitrogen. One of the oxygens is the exception and has only two lone pairs a since it has a double bond co...
A Lewis electron dot diagram (or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. ... With nitrogen, which has three p electrons, we put a single dot on each ...
Draw The Electron Dot Diagram And Structure Of A Hydrogen B Chlorine C Oxygen D Nitrogen Chemistry Topperlearning Com J0tirjrr
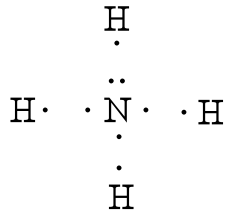
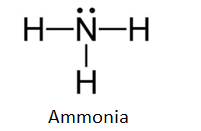
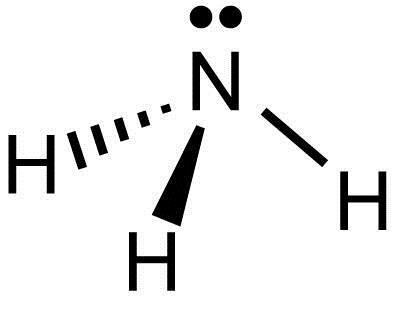
Lewis Structure (electron dot diagram) for ammonia OR Note that there are 3 covalent bonds (3 bonding pairs of electrons) in total, and that there is a lone pair (non-bonding pair) of electrons on the nitrogen atom.
The Lewis structure for NH3 is.The Lewis dot structure for NH3 starts with an N atom connected on three sides with a dash, each to an H atom. The N atom then has two dots on the unconnected side. NH3, commonly known as ammonia, is arranged as a T-shaped molecule with nitrogen at its center and three hydrogen atoms at its extremities.
Draw the electron dot structure of Nitrogen molecule [N = 7] Medium. Open in App. Solution. Verified by Toppr. Nitrogen molecule N = 7 ...
Nitrogen (N 2) Molecule Lewis Structure. Nitrogen is a diatomic molecule and contains only two nitrogen atoms. Lewis structure of N 2 molecule contains a triple bond and each nitrogen atom has one lone pair. There are many things to learn when we draw N 2 lewis structure.. N 2 lewis structure. There is a triple bond between nitrogen atoms and one lone pair exist on each nitrogen atom.
A step-by-step explanation of how to draw the NI3 Lewis Dot Structure (Nitrogen Triiodide).For the NI3 structure use the periodic table to find the total num...
Which Of The Following Is The Correct Representation Of The Electron Dot Structure Of Nitrogen Sarthaks Econnect Largest Online Education Community

Difference Between Lewis Dot Symbol And Lewis Structure Compare The Difference Between Similar Terms

Difference Between Lewis Dot Symbol And Lewis Structure Compare The Difference Between Similar Terms













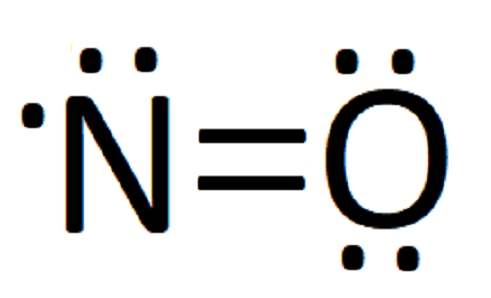






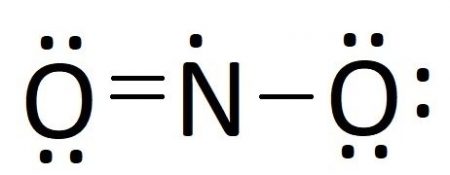



0 Response to "41 electron dot diagram for nitrogen"
Post a Comment