40 c2 molecular orbital diagram
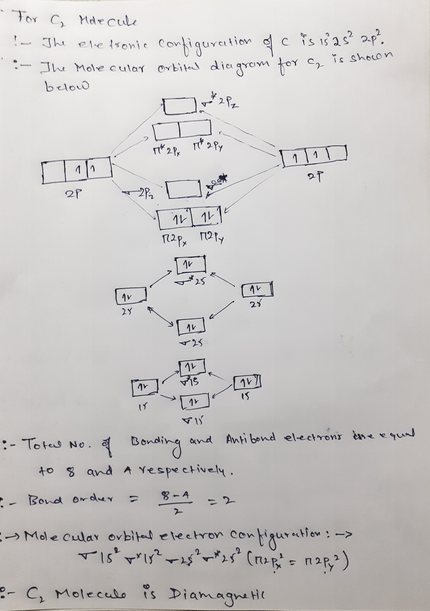
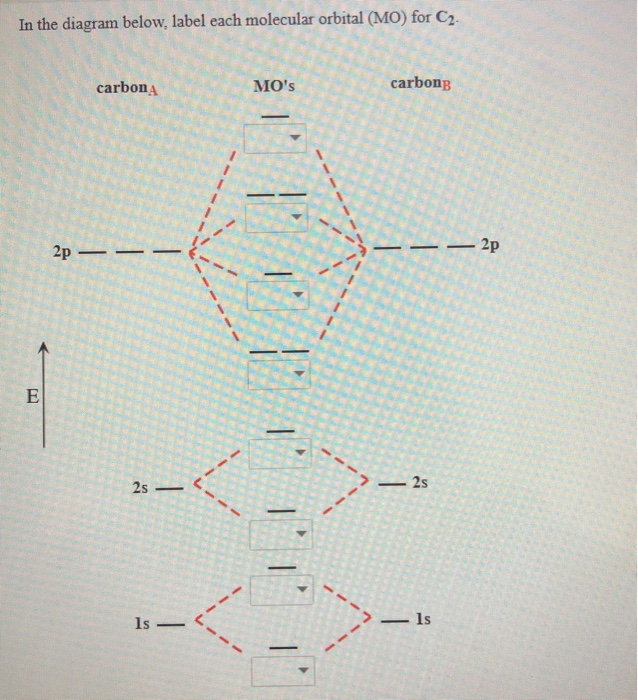
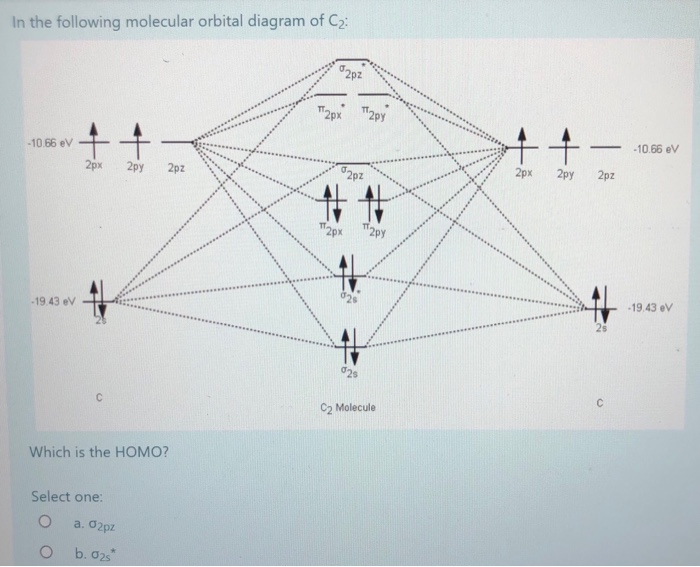
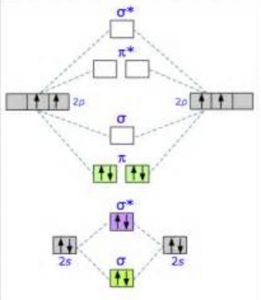
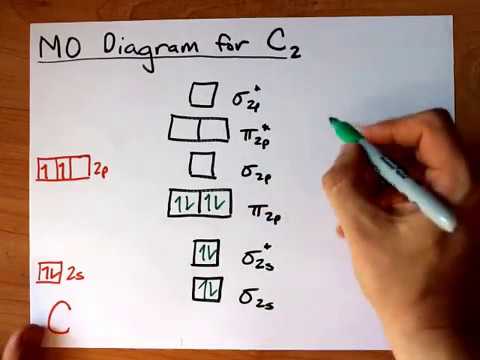
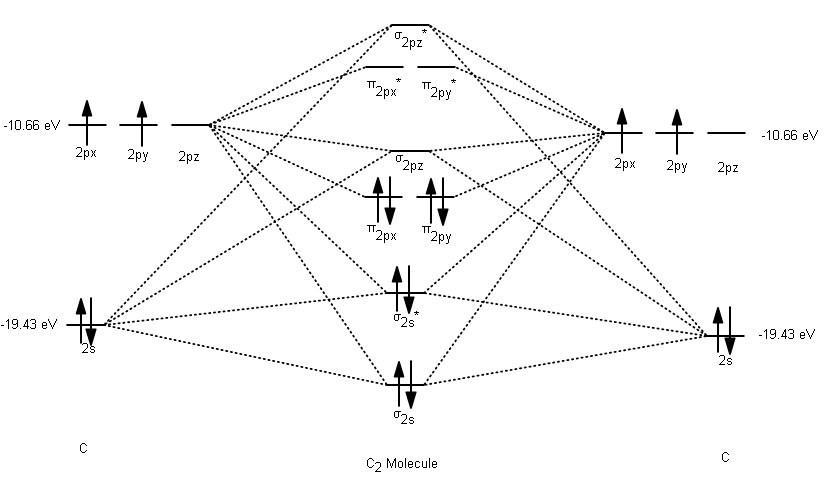
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Molecular orbital diagram for c2 2-. Summary MO Theory • LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. • Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals. • Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. • Next we'll see that symmetry will help us treat larger molecules in
Molecular orbital diagram for b2. B2 molecular orbital diagram. This also causes a large jump in energy in the 2p σ orbital. For example when two hydrogen atoms bond a σ1s bonding molecular orbital is for med as well as a σ1s antibonding molecular orbital. Valence bond model vs. The molecular orbital diagram for an o 2 molecule would there ...
C2 molecular orbital diagram
Molecular orbital diagram for carbon dimer c2. For the ion c22. Give the molecular orbital configuration for the valence electrons in cec22. A draw the molecular orbital diagram. N 2 has a bond order of 3 and is diamagnetic. Bonding order is 2 and it is diamagnetic. Interact and form molecular orbitals. B calculate the bond order. The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons. Draw the molecular orbital diagram for C. The number of electrons in the C2, molecular orbital is 1 6.2 6.3 d. 4 e zero 14. Draw the molecular orbital diagram for N. The number of electrons in antibonding molecular orbitals is a. 6 b. 2 c. 10 d. 4 e. zero 15. Draw the molecular orbital diagram for N.
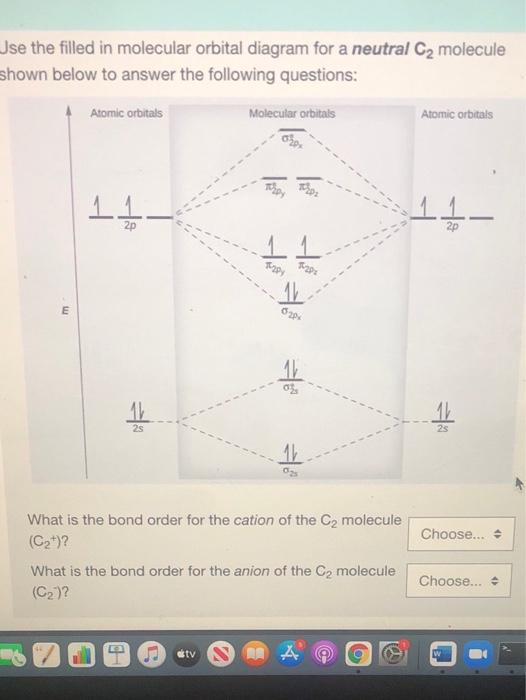
C2 molecular orbital diagram. Re: C2+ C2- Therefore, the 8 electrons would fill up both outer orbitals, the s and p orbitals, while for C2 - it would only fill up the 1s orbital and have 2 electrons in the 2s orbital. Therefore, C2 - has a stronger bond as it is more stable and harder to pull an electron away from it. Answer (1 of 2): Here is the MO diagram for O₂: Whilst this is the MO diagram for N₂: If we compare such diagrams for the diatomic molecules on the Second Period (Li₂, Be₂, B₂, C₂, N₂, O₂, and F₂), the resulting pattern looks like this: When it comes to O₂ and N₂, I think there are two things ... Molecular orbital diagram for c2. This video shows the mo diagrams of the c2 n2 o2 and f2 molecules. Molecular orbitals are formed combining similar atomic orbitals. Just because some chemical species shows integral value of bond order doesnt mean that it should exist. Molecular orbital diagram for the molecule oxygen o2. Molecular Orbitals of the Second Energy Level. The 2s orbitals on one atom combine with the 2s orbitals on another to form a 2s bonding and a 2s * antibonding molecular orbital, just like the 1s and 1s * orbitals formed from the 1s atomic orbitals. If we arbitrarily define the Z axis of the coordinate system for the O 2 molecule as the axis along which the bond forms, the 2p z orbitals on the ...
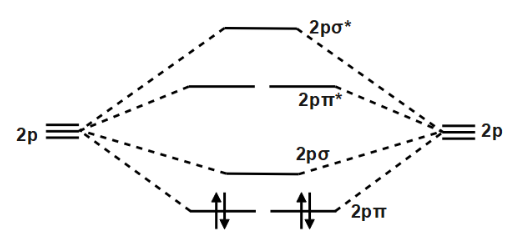
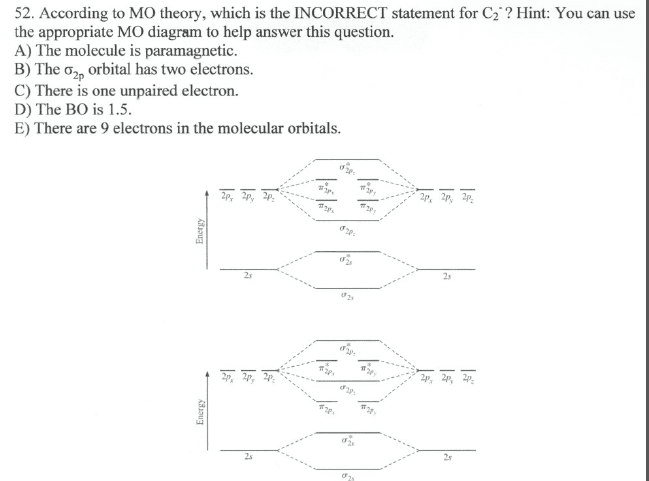
The diagram below shows the two $ 2p\pi $ orbitals, let's say $ 2p\pi x $ and $ 2p\pi y $ , are the highest energy occupied molecular orbitals. The lowest energy unoccupied molecular orbital is $ 2p\sigma $ , so that is where extra electrons will be added. Molecular Orbital Diagram - Cl2, Br2, I2 3s & 3p and higher atomic orbitals are not so widely separated in energy and allow significant mixing (hybridization) to occur. This mixing causes the inversion of the σσand πmolecular orbitals' energy. σσσ ππ σ* π* 3,4,5 p 3, 4,5 s σ* σ 3,4,5 s 3,4,5 p Interhalogens Br Br F F Br F F F F. Molecular orbital theory describes the distribution of electrons in molecules in much the same way that the distribution of electrons in atoms is described using atomic orbitals. Using quantum mechanics, the behavior of an electron in a molecule is still described by a wave function, Ψ, analogous to the behavior in an atom.Just like electrons around isolated atoms, electrons around atoms in ... Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation and the nitrosyl anion 1 Order of filling of molecular orbitals in heteronuclear diatomic molecules such as CO. Mar 26, · This video shows the MO diagrams of the C2, N2, O2 and F2 molecules.A molecular orbital (MO) energy level diagram - Parkway C-2Use the molecular orbital ...
Relationship between electronic configuration and Molecular behaviour. 1) Stability of molecules in terms of bonding and antibonding electrons . Number of electrons present in the bonding orbitals is represented by N b and the number of electrons present in antibonding orbitals by Na.. 1) If N b > Na,the molecule is stable because greater number of bonding orbitals are occupied than ... Molecular Orbital Diagram For Li2. This is the molecular orbital diagram for the homonuclear diatomic Be2+, . electrons would be in a bonding orbital, we would predict the Li2 molecule to be . Learn to draw molecular orbital electron configuration energy diagrams. molecular orbital electron configuration diagram for Li2 (Figure "Molecular ... Figure A partial molecular orbital energy-level diagram for the HF molecule. This interaction introduces an element of s-p mixing, or hybridization, into the molecular orbital theory. The result is a slight change in the relative energies of the molecular orbitals, to give the diagram shown in the figure below. The lowest energy unoccupied molecular orbital is 2pσ, so that is where the extra electron will be added. The electron configuration of the neutral C2 molecule is -- I'll use the notation given to you in the diagram. C2:(1sσ)2(1s* σ)2(2sσ)2(2s* σ)2(2pπ)4. The electron configuration of the C− 2 ion will be.
Answer (1 of 5): C2 exists, but only above 3,642 °C (6,588 °F) i.e. in vapor state
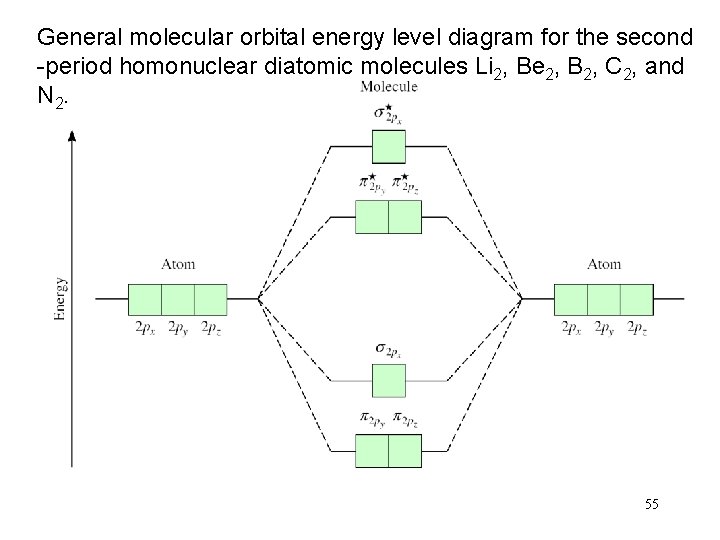
A molecular orbital diagram, or MO diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) method in particular. A fundamental principle of these theories is that as atoms bond to form molecules, a certain number of atomic orbitals combine to form the same number of ...
Printable O2 molecular orbital diagrams are available for you to guide your study in the molecular orbital lesson.This diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of a molecular orbital theory in general and the linear combination of atomic orbitals (LCAO) molecular orbital method in particular.
Diatomic carbon (systematically named dicarbon and 1λ 2,2λ 2-ethene), is a green, gaseous inorganic chemical with the chemical formula C=C (also written [C 2] or C 2).It is kinetically unstable at ambient temperature and pressure, being removed through autopolymerisation. It occurs in carbon vapor, for example in electric arcs; in comets, stellar atmospheres, and the interstellar medium; and ...
This diagram should be to scale. If necessary, you can omit the two lowest molecular orbitals from the diagram. Write the molecular orbital configuration for your molecule. Calculate the bond order for your molecule. Jen B2 Jeff OH- Mike F C2 Laura Li2 Jill Be2 Cody N2 Diana CO Kevin BF Wai BH Mike S NH Ly HF Jake SH Melissa F2
When two carbons atoms bond, the pi(2p) bonding molecular orbitals are lower in energy than the sigma(2p) bonding orbitals.C2(2-) has a bond order of 3, so i...
C2 molecular orbital diagram. A mo is defined as the combination of atomic orbitals. As for bond orders it is 12e in bonding orbitals e in antibonding orbitals. The only orbitals that are important in our discussion of molecular orbitals are those formed when valence shell orbitals are combined.
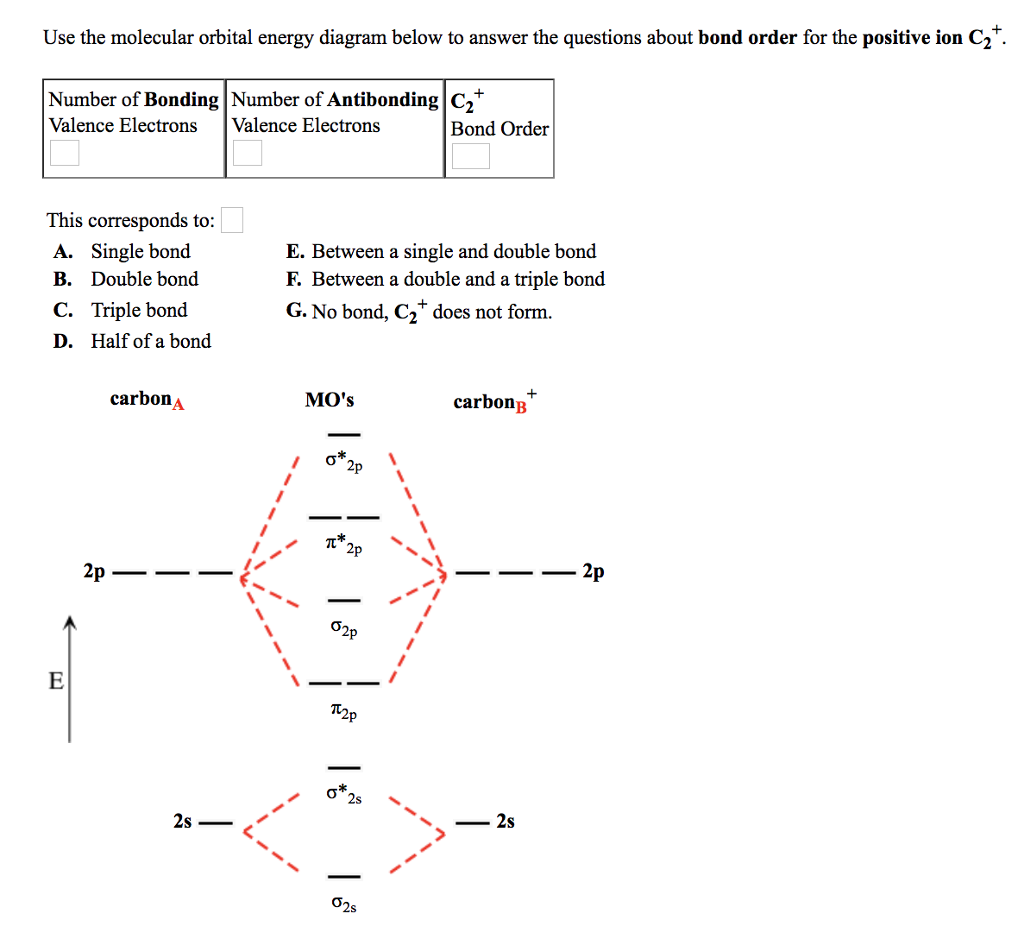
Solved 5 Draw Complete Molecular Orbital Diagrams To Compare The Bonding In C2 F2 And Cf A What Is The Bond Order Of Each B Which Of The Thre Course Hero
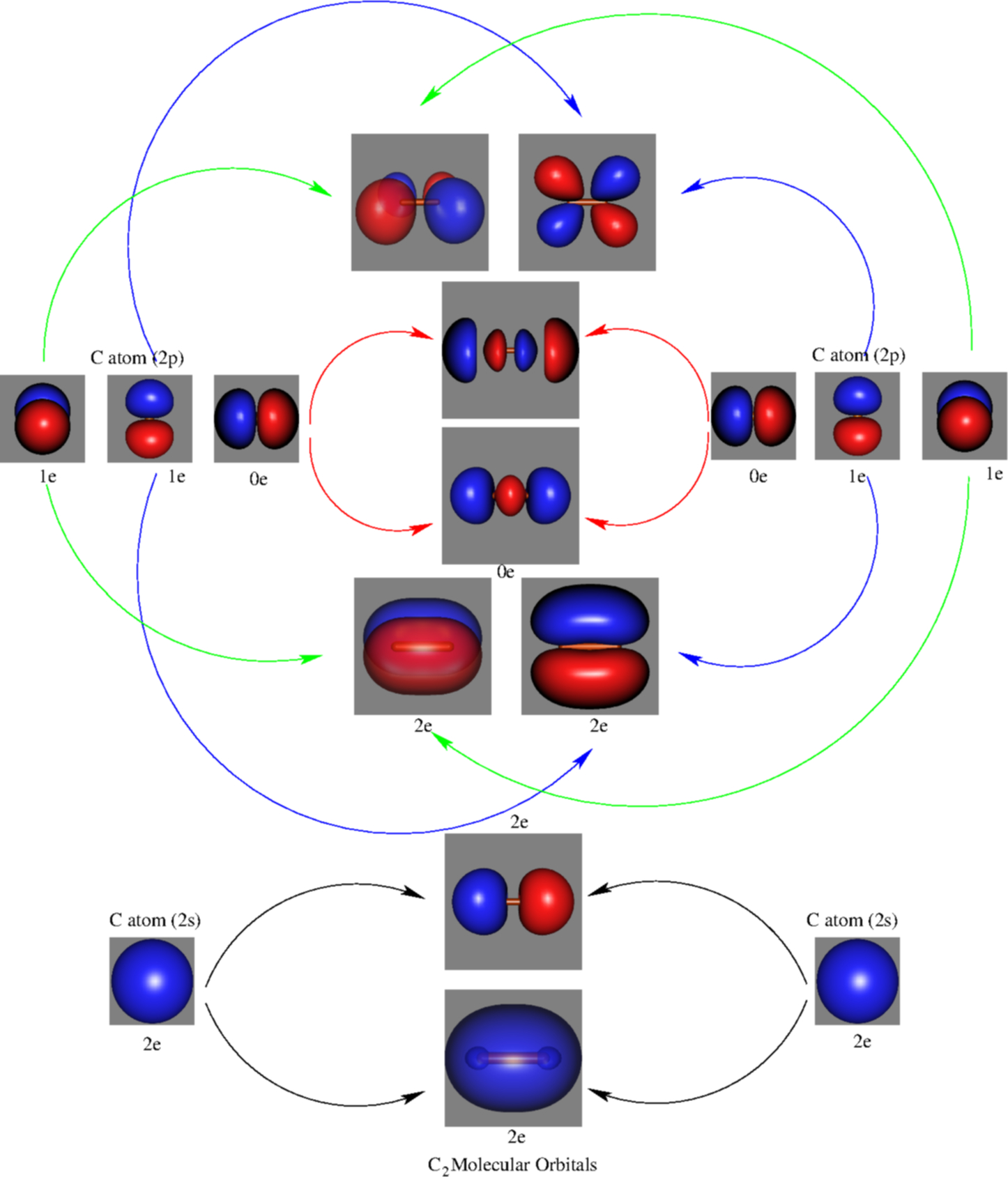
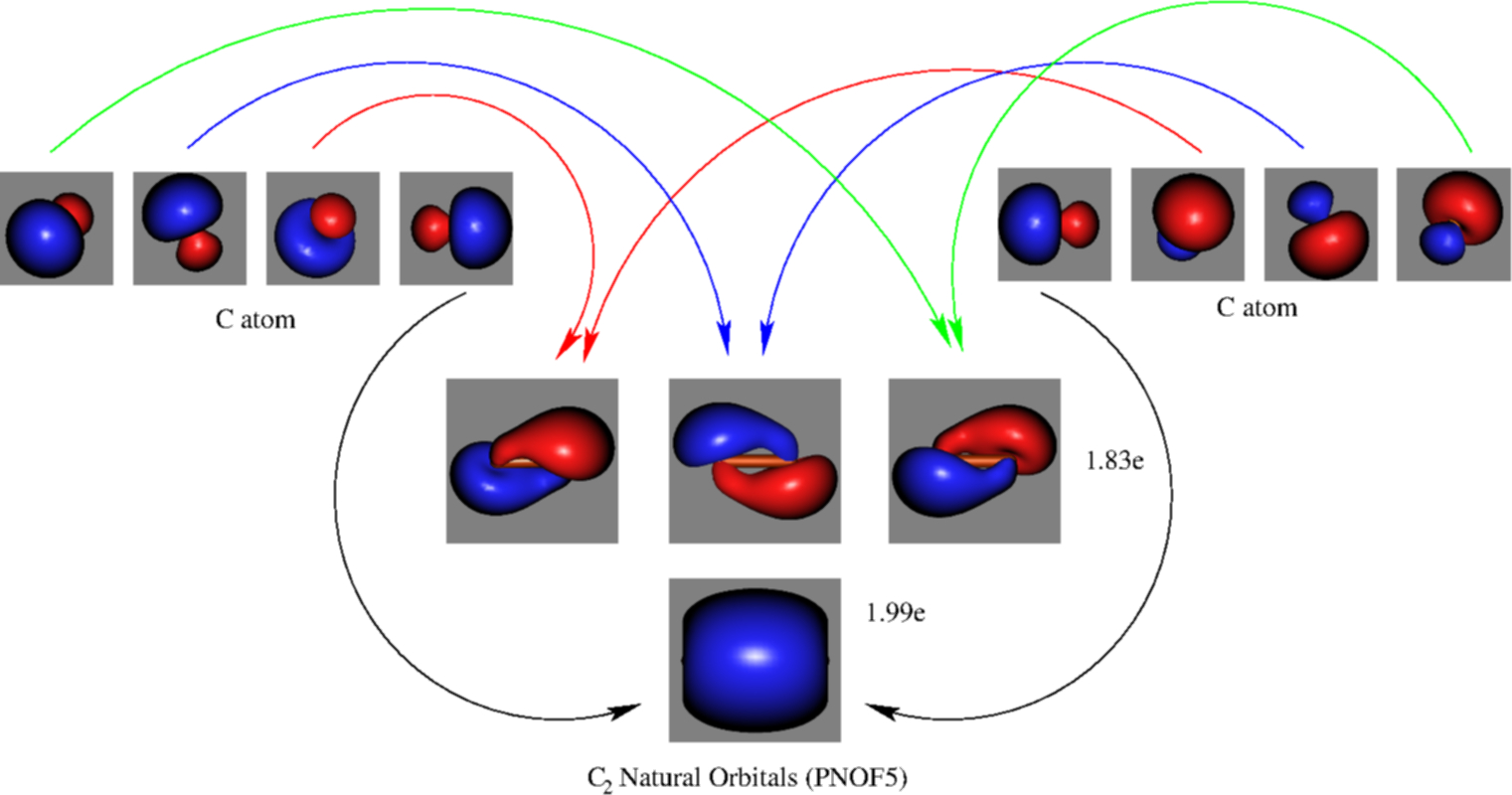
FIG. 1. The 1σg (left panel) and 1σu (right panel) molecular orbitals of C2 The next two molecular orbitals (2σg and 2σu) corresponds to the symmetric and, re-spectively, antisymmetric linear combinations of the 2s orbitals on each C atom. Note that the 2σu orbital also contains a substantial contribution from the 2pz (l = 0) orbitals (the 2p
Before we can draw a molecular orbital diagram for B₂, we must find the in-phase and out-of-phase overlap combinations for boron's atomic orbitals. Then we rank them in order of increasing energy. We can ignore the 1s orbitals, because they do not contain the valence electrons. Each boron atom has one 2s and three 2p valence orbitals. The 2s orbitals will overlap to form 2sσ and 2sσ ...
Molecular Orbital C2- Diagram, Bond Order, Magnetism. Postby Brooke Tobias 1B » Fri Nov 27, 2015 7:31 pm. A video explaining the molecular orbital diagram and notation of C2- along with the bond order and magnetism. You do not have the required permissions to view the files attached to this post. Top.
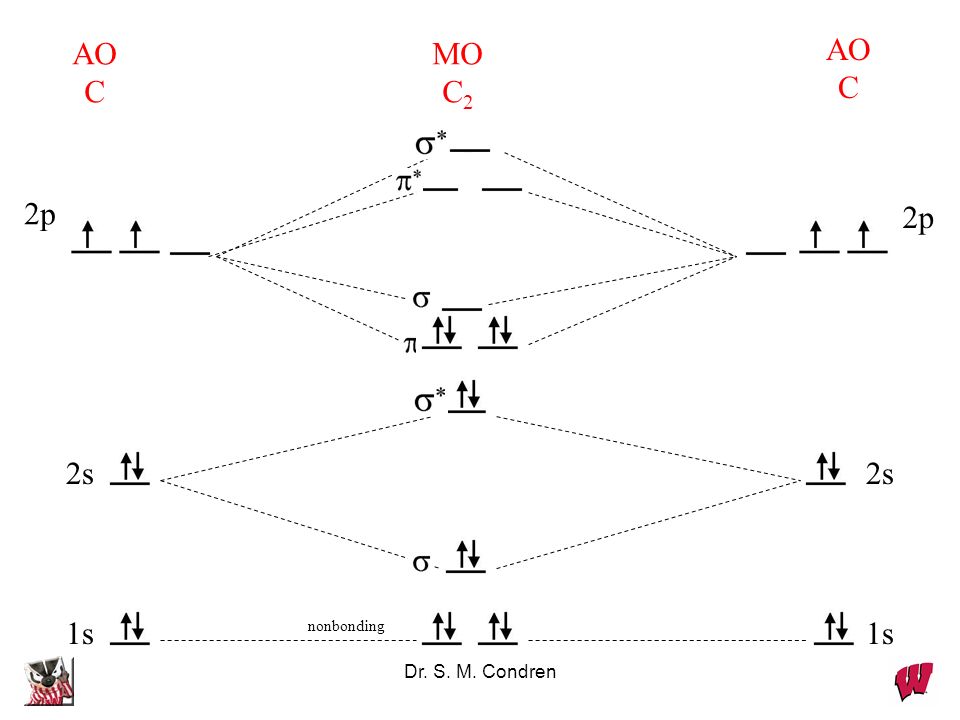
Chapter 10 Bonding And Molecular Structure Orbital Hybridization And Molecular Orbitals Dr S M Condren Ppt Video Online Download
Draw the molecular orbital diagram for C. The number of electrons in the C2, molecular orbital is 1 6.2 6.3 d. 4 e zero 14. Draw the molecular orbital diagram for N. The number of electrons in antibonding molecular orbitals is a. 6 b. 2 c. 10 d. 4 e. zero 15. Draw the molecular orbital diagram for N.
The molecular orbital diagram for C 2 molecule is :. The electronic configuration of C 2 is K K (σ2s) 2 (σ * 2s) 2 n(2px) 2 n(2py) 2. The C 2 molecule is diamagnetic because all electrons are paired there are no unpaired electrons.
Molecular orbital diagram for carbon dimer c2. For the ion c22. Give the molecular orbital configuration for the valence electrons in cec22. A draw the molecular orbital diagram. N 2 has a bond order of 3 and is diamagnetic. Bonding order is 2 and it is diamagnetic. Interact and form molecular orbitals. B calculate the bond order.
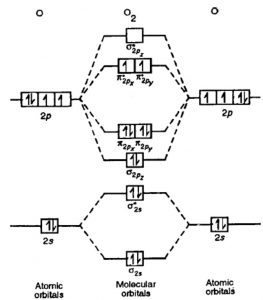
Energy Level Diagram For Molecular Orbitals Chemical Bonding And Molecular Structure Chemistry Class 11

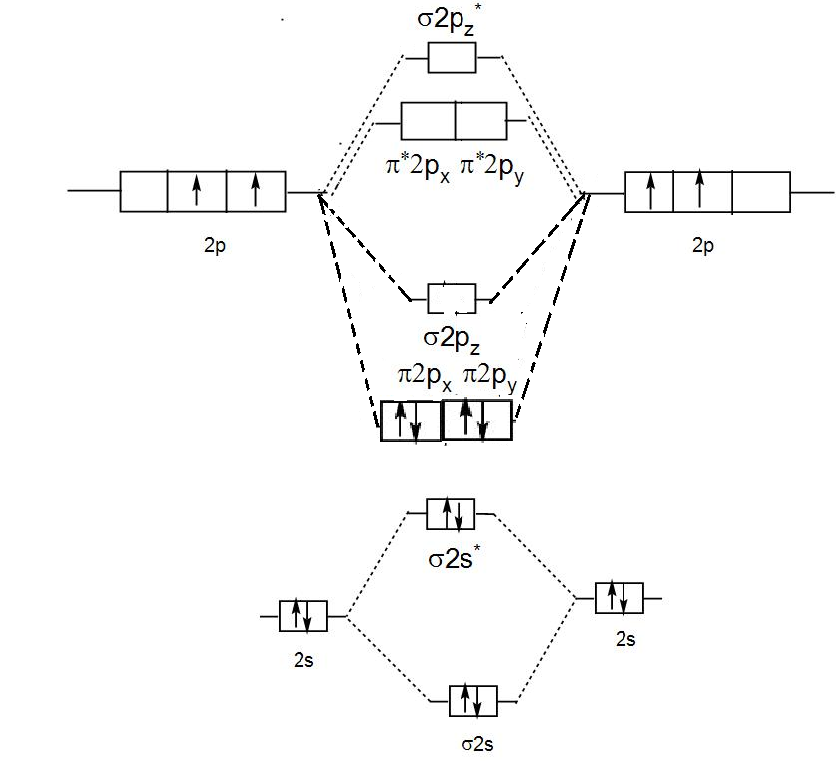


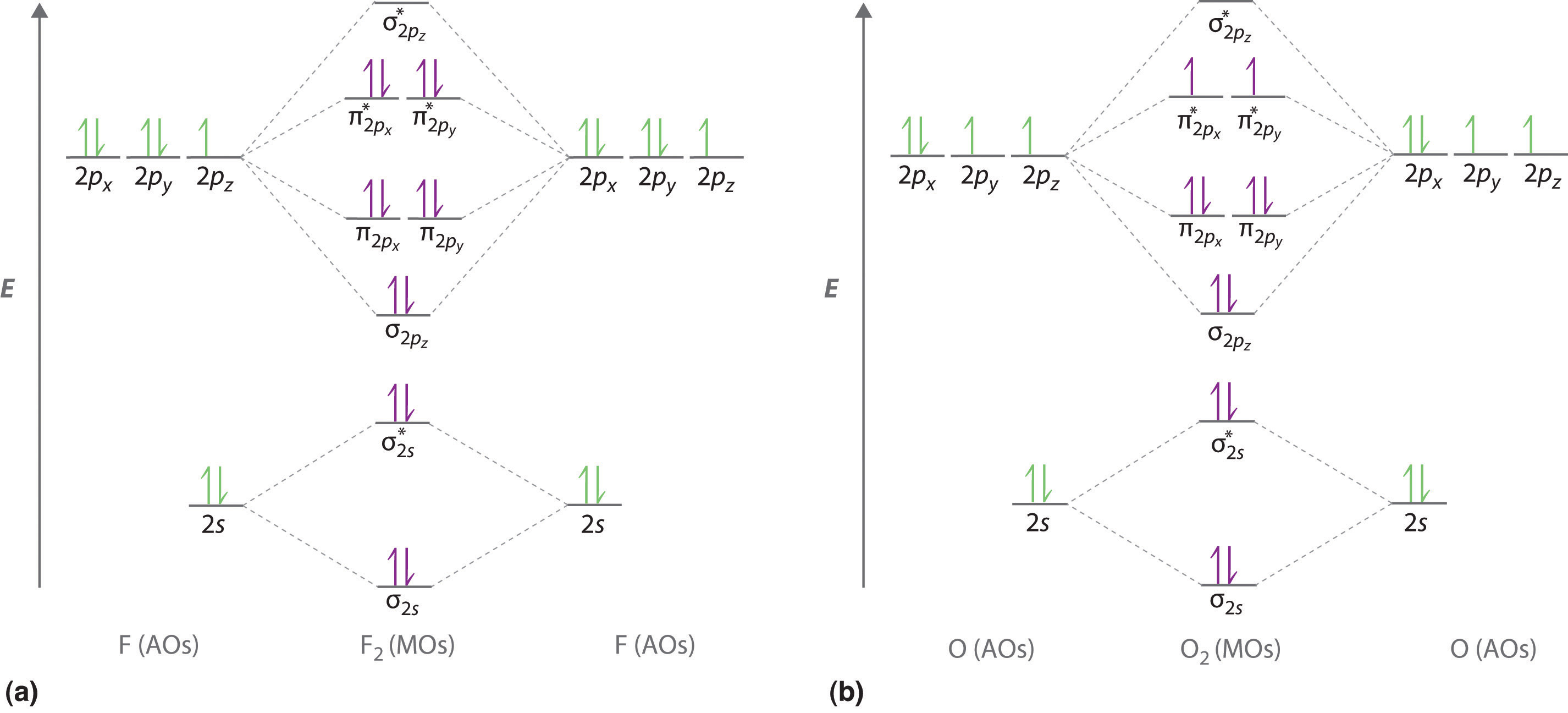








0 Response to "40 c2 molecular orbital diagram"
Post a Comment